Premature ventricular complexes (PVCs) are the most common ventricular arrhythmia. Their prognostic significance cannot be interpreted without considering the presence or absence of any associated underlying cardiac condition. In the absence of structural heart disease, PVCs were generally considered to be benign.1,2 In the 1970s and 1980s, it was postulated that frequent PVCs could be a trigger for ventricular tachycardia (VT), ventricular fibrillation (VF) and sudden cardiac death in post-MI patients, and therefore PVC suppression was thought to be warranted in this context.
In the Cardiac Arrhythmia Suppression Trial (CAST), treatment of PVCs with antiarrhythmic drugs increased mortality in patients with previous MI, despite effectively suppressing asymptomatic PVCs;3 findings attributed to the proarrhythmic effects of the drugs used. Despite the CAST trial showing a decrease in PVC burden and no mortality benefit, more recent work has revealed that PVCs can contribute to cardiomyopathy and heart failure and treating PVCs could lead to improved cardiac function.4,5 PVC-induced cardiomyopathy is a potentially reversible condition in which left ventricular dysfunction is induced by frequent PVCs and function improves on suppressing PVCs.
Our aim is to review the underlying mechanisms and risk factors associated with the development of PVC-induced cardiomyopathy, and compare the indications and effectiveness of the interventional and medical treatment options.
Epidemiology, Prevalence and Prognosis
In a normal healthy population, PVCs have been observed in up to 75 % of subjects on 48-hour Holter monitoring,6 with >60 PVCs/hour detected in up to 4 % of individuals.2 This latter prevalence increases progressively with age, comorbidity burden and duration of monitoring, ranging from 1–69 %.7,8
The adverse impact of frequent PVCs on prognosis in patients with underlying or structural cardiac disease, such as previous MI, is well established.9 In the late 1990s, Duffee et al. demonstrated that pharmacological suppression of PVCs in patients with presumed idiopathic dilated cardiomyopathy subsequently improved left ventricular ejection fraction (LVEF).10 Recent studies have demonstrated the potential detrimental effects of frequent PVCs in patients with structurally normal hearts and the development and reversibility of PVC-induced cardiomyopathy.4,5 Frequent PVCs can also worsen a pre-existing cardiomyopathy, in which case PVC suppression may only lead to partial recovery of the LV dysfunction.11
A PVC burden >24 % has been suggested as having the highest sensitivity and specificity (79 % and 78 %, respectively) to predict the occurrence of PVC-induced cardiomyopathy.5 However, a recent study has shown that heart failure may be caused by a much lower PVC burden than that traditionally associated with PVC-induced cardiomyopathy.12 Further studies are necessary to clarify why cardiomyopathy can develop with such a low PVC burden.
Mechanisms and Pathophysiology
Tachycardia-induced cardiomyopathy was originally considered to be the underlying mechanism of PVC-induced cardiomyopathy.4,13 However, the exact underlying mechanism is not entirely clear, as many patients with frequent PVCs and cardiomyopathy have similar average heart rates when compared with individuals without PVCs and/or cardiomyopathy.13,14
From a cellular perspective, the mechanisms of PVC-induced cardiomyopathy are speculative and based on animal models, from which extrapolation for humans is sometimes limited. In their assessment of a canine model, Wang et al. postulated that the prolongation and marked beat-to-beat variation in action potential duration, as well as decreased outward and inward (L-type calcium) currents, could result in increased repolarisation heterogeneity.15 This may be associated with an increased risk of sudden cardiac death due to triggered activity and malignant ventricular arrhythmias. They also postulated that the contractile dysfunction observed in PVC-induced cardiomyopathy could be explained by an altered calcium-induced calcium release from the sarcoplasmic reticulum. In another canine model,16,17 it was reported that LVEF impairment could occur within 3 months of induced ventricular ectopy. This suggests that the underlying mechanism is functional rather than structural, given the absence of myocardial fibrosis and changes in apoptosis.
From a clinical perspective, the mechanical ventricular dyssynchrony resulting from the abnormal electrical ventricular activation may be a more straightforward explanation.18,19 Ventricular dyssynchrony may contribute to LV impairment in the same way as it has been described in the context of left bundle branch block, either physiological or induced by chronic right ventricular pacing, asymmetrically increased wall thickness in the late-activated regions and altered myocardial blood flow.20–22
Risk Factors for the Development of Cardiomyopathy
Not all patients with PVCs will go on to develop a cardiomyopathy. Indeed, some patients with high burdens of PVCs remain free from symptoms and never seem to develop any LV dysfunction. Factors that have been suggested to influence the development of PVC-induced cardiomyopathy are discussed below.
Premature Ventricular Complex: QRS Features, Interpolation and Coupling Intervals
A PVC QRS duration ≥140 ms has been reported as an independent predictor of LVEF impairment,23–25 which is more commonly observed in PVCs originating from the free wall and outflow tracts. Those with a narrower QRS typically originate from the septum or fascicles. The presence of interpolated PVCs has also been reported as predictive of PVC induced cardiomyopathy. In a single-centre, small study, both the occurrence of interpolated PVCs and the burden of PVCs associated with a higher risk of PVC-induced cardiomyopathy.26
PVC coupling intervals ≤600 ms are associated with a lower mean LVEF, possibly due to an abnormal filling of the LV and decreased stroke volume.27,28 A coupling interval variability of 60 ms was found to be more frequent in PVCs originating from the sinus of Valsalva or the great cardiac vein and may be associated with increased frequency of cardiac events.29 However, this is yet to be confirmed in larger studies.
Premature Ventricular Complex Burden
A high PVC burden is one of the factors thought to predispose a person to the development of cardiomyopathy. However, not every patient with frequent PVCs develops cardiomyopathy. An increased long-term risk of incident chronic heart failure (CHF) and death has been reported in patients with high density of PVCs, suggesting that PVCs may represent a modifiable risk factor for CHF.12 Some studies have shown a correlation between the burden of PVCs and the severity of the LV impairment.14,18 A cut-off point of 24 % has been proposed as having the best sensitivity and specificity for the prediction of cardiomyopathy with a sensitivity of 79 % and specificity of 78 %.5 However, we cannot currently consider a clear cut-off value, as PVC-induced cardiomyopathy can be observed even in patients with lower PVC burden (>10 %)4,5,12 and people with high PVC burden could be completely asymptomatic with no LV dysfunction. We also need to take into account that 24-hour Holter monitoring may be insufficient to accurately characterise the real PVC burden in some patients and may influence studies when assessing cut-off values for PVC percentage.30
Origin
PVCs originating from the ventricular outflow tract musculature, and especially those from the right ventricular outflow tract (RVOT), represent two-thirds of idiopathic PVCs.23 These include those arising from myocardial extensions to the aortic and pulmonary cusps. The remaining third originate from different locations (septum, papillary muscles, free walls or left ventricular fascicles). The typical outflow tract arrhythmia pattern on the surface 12-lead ECG includes an inferior axis, characterised by a positive QRS in leads II, III and aVF. A left bundle branch block-like morphology often suggests an RVOT origin, although an aortic cusp origin may also present like this, albeit with earlier QRS transition. On the other hand, a right bundle branch block-like morphology typically suggests a left-sided focus. However, the anatomy of the outflow tracts has complex 3D anatomical relationships, with the RVOT located leftward and anterior to the LVOT and the pulmonary valve superior to the aortic valve. As such, there are different and subtle ECG differences that may suggest alternative anatomical locations of the PVCs:
- A QRS transition in the precordial leads later than that in sinus rhythm suggests an RVOT exit (and vice versa), as the more anterior structure, the later precordial transition.31,32 If the QRS transition in both PVC and sinus beats is at V3, the “R wave transition ratio” can provide further guidance. When comparing the PVC R-wave amplitude in V2 with that in sinus rhythm, a ratio ≥0.6 predicts a left-sided origin with a sensitivity of 95 % and a specificity of 100 %.33
- The maximum deflection index, defined as the ratio between the time to maximum deflection and the QRS duration, can help determine whether the PVC focus is epicardial. A value above 0.55 has been suggested as predictive of an epicardial origin.34
These apparently subtle ECG differences become key when evaluating a patient for the first time in the clinic and planning an ablation procedure. Different PVC locations may require different vascular access and are associated with different rates of success and complications.
Del Carpio et al. found that right ventricular (RV) PVCs could cause LVEF impairment at a lower daily burden than those originating in the LV (10 % versus 20 % burden, respectively).23 This may be due to the increased LV dyssynchrony potentially associated with RV PVCs compared with those originating from the LV. This was a small study so this finding should be interpreted with caution. A more recent study has postulated that an epicardial origin may associate with the highest risk for developing cardiomyopathy. As before, a possible explanation is the greater degree of mechanical ventricular dyssynchrony seen with epicardial PVCs.19
Circadian Variability
The consistency in PVC burden throughout the day has been reported as an independent predictor of PVC-induced cardiomyopathy.35 A recent study in patients with frequent monomorphic PVCs referred for radiofrequency ablation (RFA) hypothesised that PVC circadian variation may help predict PVC inducibility in the electrophysiology lab, facilitating the success of the ablation procedure.36 In fact, those patients with fast heart rate dependent PVCs had the highest successful outcomes from RFA as they responded to isoproterenol during the procedure, while patients with no correlation between PVCs and mean heart rate had the least successful outcomes.
Gender
A large study by Latchamsetty et al. suggested that male sex may be an independent risk factor for the development of cardiomyopathy.37,38 Surksha et al. found that the incidence of symptomatic PVCs was greater in women, while that of idiopathic ventricular tachycardia was similar among sexes.39 Considering that asymptomatic status could delay the diagnosis and hence facilitate the development of a cardiomyopathy, women may be less prone to develop cardiomyopathy as they will be treated at an earlier stage. This sex-related variation may, in part, be secondary to hormonal differences, but may also be related to differences in symptom perception – women may be more sensitive to PVCs and seek medical attention sooner than men.40
Clinical Presentation and Initial Approach
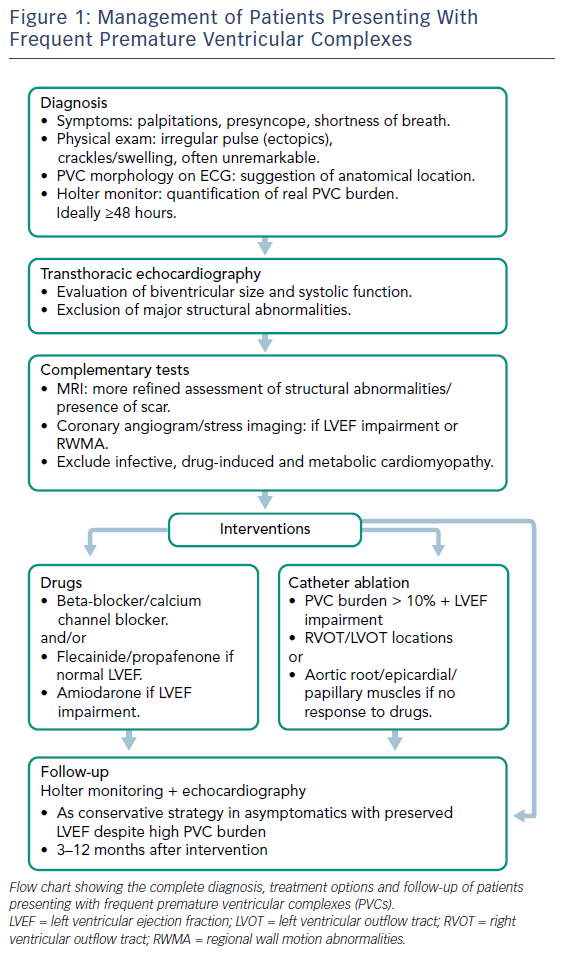
Identifying the primary disorder is essential due to the potential reversibility of PVC-induced cardiomyopathy. However, it can be difficult to determine whether the PVCs preceded the cardiomyopathy or were a result of it. Therefore, PVC-induced cardiomyopathy is often a diagnosis of exclusion after ruling out other potential causes of cardiomyopathy.
Symptoms and Initial Evaluation
In the acute setting, the most frequent symptoms related with PVCs are palpitations, either secondary to the PVCs themselves or due to the increased stroke volume of the post-PVC beat. The latter hypothesis has been challenged recently.41 Patients may also present with shortness of breath, pre-syncope/syncope and chest pain/discomfort.
The cumulative haemodynamic effect of frequent PVCs means that in the chronic setting, symptoms can range from different degrees of functional deterioration to manifest decompensated heart failure as a result of decreased effective cardiac output. This scenario includes patients receiving cardiac resynchronisation therapy (CRT) with sub-optimal CRT response or lack thereof, in whom the role of PVCs as the trigger for the progressive cardiomyopathy might have been underestimated.41
Nevertheless, some patients are asymptomatic and diagnosed incidentally during a routine check. Asymptomatic presentation may be a risk factor for PVC-induced cardiomyopathy as the diagnosis of the arrhythmia could be delayed and subsequently lead to cardiomyopathy.19,42 It is important to emphasise that many patients with PVCs are asymptomatic and have preserved LV function. In these patients, there could be a considerable duration between the incidental diagnosis of PVC and development of LV dysfunction and some may never develop any symptoms or cardiomyopathy.
The physical examination is often unremarkable in patients without heart failure, except for the irregular pulse caused by the ectopic beats. An ECG is essential to assess PVC morphology and estimate the location of the PVC foci, particularly in patients who are referred for catheter ablation. The PVC burden is best assessed by continuous Holter monitoring, ideally for 48–72 hours to avoid the misleading effect on the true PVC density that the day-to-day variability can produce in monitoring limited to 24 hours.30,41 A 12-lead Holter would be very useful, particularly in patients under consideration for ablation, in order to accurately identify the number of PVC morphologies.
Complementary Tests
Transthoracic echocardiography is mandatory to exclude other causes of PVCs such as valvular or ischaemic heart disease, and for the assessment of LV impairment. The most common echocardiographic findings in PVC-induced cardiomyopathy include increased systolic and diastolic LV size, with global rather than regional LV systolic impairment (2D speckle tracking strain might show altered LV contractility despite normal LVEF43), and functional mitral regurgitation. However, it should be considered that LVEF may be difficult to assess in people having incessant PVCs or bigeminy and attempts should be made to assess the LVEF during cardiac cycles where no PVCs are observed.
Cardiac MRI with gadolinium is a useful technique to evaluate the presence of scar and rule out infiltrative diseases, as well as for the detection of arrhythmogenic right ventricular cardiomyopathy, with or without LV involvement.37 A pre-procedural cardiac MRI is helpful in planning an ablation procedure and might help select appropriate candidates for catheter ablation, whether they have ischaemic or non-ischaemic cardiomyopathy, or even if there is no evidence of structural heart disease.44,45 One study of 162 patients presenting with palpitations and documented exercise-induced PVCs, but no evidence of structural heart disease, found that cardiac MRI showed evidence of myocardial disease consistent with acute or previous myocarditis or myopericarditis in the majority of those patients.46 In general, it is likely that imaging modalities may be more helpful in the future by defining more suitable candidates for intervention.
Coronary angiography or a CT angiogram, depending on the cardiovascular risk profile, should be performed in every patient with impaired LV systolic function to exclude significant coronary artery disease.
As a diagnosis of exclusion, PVC-induced cardiomyopathy requires the exclusion of other causes of cardiomyopathy, such as infective, drug-induced and metabolic, if imaging tests are inconclusive. Other possible triggers for PVCs, such as excess alcohol/caffeine intake or emotional stress, must also be excluded (Figure 1).
Treatment Options and Management
Treatment is usually indicated in patients with debilitating symptoms, LV systolic dysfunction, malignant ventricular arrhythmias triggered by PVCs and suboptimal biventricular pacing in those with CRT. In general, treatment includes management of secondary causes, pharmacotherapy to suppress PVCs, or catheter ablation to reduce or eliminate PVCs. The reduction of caffeine and alcohol intake and a better control of emotional stress have modest effectiveness in reducing PVC frequency.47 At present, there is no evidence to support that asymptomatic patients with frequent PVCs and preserved LVEF should be considered for any specific treatment, though beta-blockers or calcium channel blockers (CCB), as tolerated, could be discussed. Such patients should have their LVEF assessed at regular intervals and they should be advised to report if they develop symptoms of heart failure. Despite the fact a high PVC burden is a key factor for developing cardiomyopathy, it is noteworthy that the majority of patients presenting with frequent PVCs have a preserved LVEF and will not develop cardiomyopathy, suggesting a differential susceptibility among individuals despite similar PVC burden.4,14
Pharmacotherapy
In the presence of symptoms, the first line of pharmacological treatment is usually a beta-blocker. In a randomised, double-blinded, placebo-controlled study, atenolol significantly decreased symptom frequency, PVC count and average heart rate compared with placebo.48 In patients with slow baseline heart rate, or in those with increased PVC burden due to bradycardia, beta-blockers with intrinsic sympathomimetic activity may be particularly helpful.49 Alternatively, in patients intolerant to beta-blockers and with no heart failure, a non-dihydropyridine CCB may be considered, given the relatively low adverse effect profile. Reported efficacy of beta-blockers or CCB is in the range of 20 %, but they are reasonable first-line options due to their relative safety and additional symptomatic benefit provided by the dampening of hypercontractile compensatory beats following a PVC.19
The second line of treatment to consider is the use of antiarrhythmic drugs (AADs), such as flecainide, propafenone, sotalol or mexiletine.50,51 Class I and III AADs have been reported to achieve higher rates of PVC reduction (≥70 % in more than 90 % of patients taking flecainide, and in 55 % of patients on mexiletine) than beta-blockers or CCB.51,52 Class I AADs were usually contraindicated in patients with LV dysfunction or significant structural heart disease.3 However, in a small cohort of patients with suspected PVC-induced cardiomyopathy and at least one previous unsuccessful ablation procedure and then treated with Class IC AADs, PVCs were effectively suppressed with no adverse events in the follow-up period.53 In this cohort, the mean PVC burden decreased significantly and LVEF improved (including LVEF improvement in seven patients with myocardial delayed enhancement on cardiac MRI, all with less than 5 % of the total myocardium). There were no sustained ventricular arrhythmias or sudden cardiac deaths reported during an average treatment near to 4 years.
Class IC drugs have been contraindicated in people with cardiomyopathy because of the increased mortality seen in the CAST trial.3 Hyman et al. suggest that this increased mortality could be related to the interaction of Class IC AADs with residual ischaemia.53 In a substudy of the CAST trial, increased mortality was seen in people with non Q wave MI and people with both non Q wave MI and angina were more likely to die, suggesting an association between ongoing ischaemia and electrical instability.54 Thus it is possible that ongoing ischaemia rather than structural heart disease increases the risk of mortality with the use of class IC drugs. Hence in people with no ongoing ischaemia, a class IC AAD may be used, though further studies are required.
Amiodarone has shown to effectively suppress PVCs and improve LVEF.55 However, its long-term use is limited by its adverse effect profile. Dronedarone is a reasonable alternative to amiodarone, but is contraindicated in patients with recently decompensated heart failure or chronic AF.
Catheter Ablation
As with any invasive intervention, the potential benefit of catheter ablation must be weighed against the risk of major complications, estimated to occur in up to 3 % of patients.56 These include vascular complications, such as femoral pseudoaneurysm, arteriovenous fistula or groin haematoma, cardiac perforation with tamponade, intraprocedural stroke or death.57 Pharmacological alternatives, patients’ comorbidities, the anatomical location of the PVC and operator experience are factors that should be taken into account. Nevertheless, the constant improvements and innovation in ablation technology, sources of energy and advanced 3D mapping software have allowed catheter ablation to emerge as a relatively safe and effective option to eliminate or drastically reduce PVC burden and restore ventricular function. This may prevent unnecessary defibrillator insertion in patients who previously met the criteria.58 A higher prevalence of repeating forms of PVCs and shorter coupling intervals have been reported as potential risk markers for imminent ventricular tachyarrhythmia and probably justify more aggressive management.59
Catheter ablation has become a reasonable first-line option in patients presenting with RV outflow tract PVCs given the high success rate of such ablation and the low risk of complications.4,41 Other locations formerly considered as riskier, such as the aortic root or the papillary muscles, may be also safely ablated with the support of intracardiac echocardiography and electroanatomical mapping systems. At present, successful ablation of PVCs normally involve a combination of activation and pace-mapping guided by fluoroscopy, electroanatomical mapping and intracardiac echocardiography.
In patients with failed RVOT ablations, although the most frequent site of origin may still be the RVOT, consideration should be given to mapping the pulmonary artery, the coronary venous system and, importantly, the aortic cusps – especially if the earliest site of activation in the RVOT is in the posteroseptal location.60
Cryoablation may become a promising alternative to radiofrequency when ablating in certain locations, such as the left aortic root near the left main ostium, or the papillary muscle given the difficulties with catheter stability and papillary muscle mobility.61
Recovery Post-ablation
In a study to assess predictors of LV recovery and reverse remodelling in patients with frequent PVCs referred for ablation, there was a relationship between the PVC QRS duration and the probability of recovery.62 Following a successful ablation, the only predictor of lack of recovery was a broader PVC QRS duration. It was suggested that increases in PVC QRS duration may be represent more extensive underlying fibrosis, possibly contributing to the persistence of LVEF impairment. Mountantonakis et al. found that the degree of LV function recovery post-ablation in patients with true PVC-related cardiomyopathy is more pronounced than in patients with a pre-existing diagnosis of cardiomyopathy.63
In patients with a pre-existing diagnosis of cardiomyopathy and subsequent development of frequent PVCs and deterioration of LV function, successful PVC ablation may improve ejection fraction, but it is unlikely to completely normalise.44 The same study found that the arrhythmogenic substrate was located in scar tissue in most patients who underwent an effective ablation. However, reverse remodelling has been also reported in approximately half of the patients with long-term failure of the ablation procedure. This “reverse paradox” was probably due to underlying reversible cardiomyopathy.64
In patients with ischaemic cardiomyopathy and frequent PVCs, Sarrazin et al. reported that successful ablation of PVCs could increase LVEF in a significant percentage of patients. This study interestingly reported that patients with frequent PVCs had a significantly smaller scar burden by delayed enhancement MRI when compared to control patients (ventricular tachycardia patients). The presence of limited scar tissue despite severe LV impairment may suggest a superimposed cardiomyopathy because of the PVC.45
In a study by Penela et al., patients with frequent PVCs, LV dysfunction and an indication for primary prevention implantable cardioverter–defibrillator (ICD) underwent catheter ablation. They reported significant improvement of LV function over 12 months. More importantly, LV function improved enough in the majority of the patients that they did not need an ICD, without increasing the risk of ventricular arrhythmias while waiting for LV function to improve.65
To achieve adequate resynchronisation, catheter ablation would also be relevant in patients with cardiac resynchronisation device and high PVC burden.
Pharmacotherapy Versus Catheter Ablation
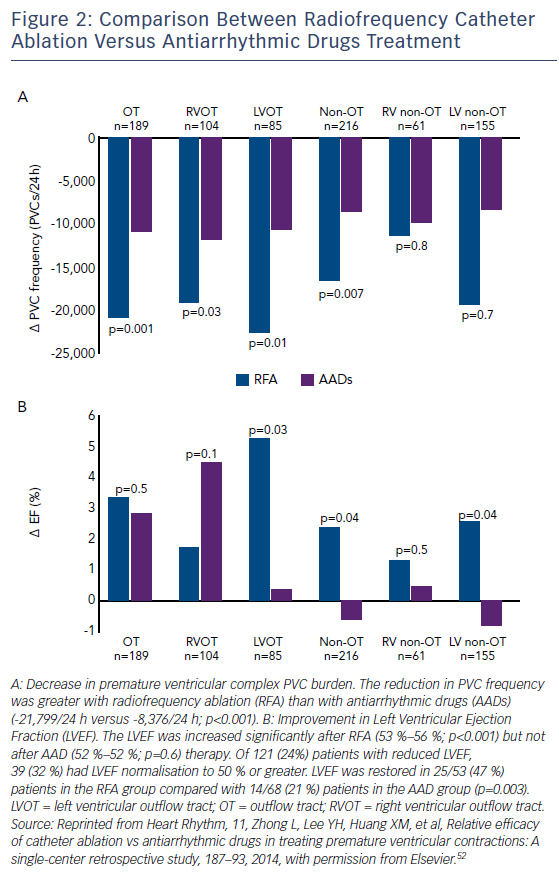
Catheter ablation is currently being evaluated as a potential first line therapy in patients with PVC-induced cardiomyopathy. Recent publications have shown that RFA is more effective than pharmacotherapy in, at least, the RVOT location, with a safe profile and a more favourable LVEF normalisation compared with AAD (Figure 2).52,66 The 2015 European Society of Cardiology guidelines state that, in patients with RVOT PVCs needing treatment, catheter ablation should be recommended as first-line treatment, whereas in patients with LVOT PVCs catheter ablation should only be considered after failed AAD.67 The 2017 American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines consider catheter ablation useful for patients who require arrhythmia suppression for symptoms or declining ventricular function suspected to be due to frequent PVCs (generally >15 % of beats and with one predominant morphology) and for whom antiarrhythmic medications are ineffective, not tolerated or refused by the patient.68
In patients with decreased LVEF, a follow-up period of 3–12 months after initiation of antiarrhythmic therapy or catheter ablation is suggested to allow for recovery of LV function and to avoid unnecessary ICD insertions in a potentially reversible condition,18,22 provided the patient does not fulfil other criteria for implantation (such as previous cardiac arrest or the occurrence of haemodynamically unstable ventricular arrhythmia). If a decision is made to implant an ICD, consideration could be given to the implantation of subcutaneous ICD, as there is usually no need for pacing in these patients and recovery of LV function is frequent following a successful ablation.
Since the LVEF is difficult to assess in patients with frequent PVCs, the question remains whether, in some cases, the LV dysfunction and subsequent improvement in LVEF post-ablation may be actually the result of inadequate assessment of LVEF. Therefore, it has been recommended that an echocardiogram should be performed immediately post-ablation to evaluate LVEF in sinus rhythm. An immediate improvement suggests a PVC related measurement issue. In contrast, the LV dysfunction should persist immediately after successful ablation but improve gradually over time in true PVC-induced cardiomyopathy.69
Conclusion
Premature ventricular complexes are commonly seen in the general population and may produce LV dysfunction and cardiomyopathy independently of any pre-existing underlying cardiac disease. The suppression of PVCs, either through medical therapy or, more effectively, catheter ablation, is indicated in symptomatic patients with frequent PVCs and also those with LV dysfunction or decreased percentage of biventricular pacing. Catheter ablation has progressively become a potential first-line therapy in patients with PVC-induced cardiomyopathy and should be strongly considered, particularly in patients with right-sided outflow tract PVCs.
There is little evidence at present to recommend treating asymptomatic patients with normal LVEF. The benefit of performing PVC ablation in these patients guided only by a high PVC burden has not yet been demonstrated and can be potentially hazardous.70
Further research is still needed to clarify the molecular, cellular and haemodynamic mechanisms of LV dysfunction in PVC-induced cardiomyopathy, as well as the risk factors for its development.