Atrial fibrillation (AF) is the most common sustained tachyarrhythmia. Catheter ablation is the most effective treatment for AF, and pulmonary vein isolation (PVI) is the cornerstone of all AF ablation strategies. The use of a radiofrequency (RF) catheter in combination with a 3D electro-anatomical mapping system is the most well established approach to AF ablation. However, manipulating a RF catheter to create continuous and effective circumferential lesions in a point-by-point fashion is challenging. Single-shot ablation systems have been developed to facilitate the ablation procedure. Of these, the cryoballoon (CB) ablation system has been investigated in the greatest depth. In recent years, balloon-based ablation has emerged as an encouraging alternative to RF ablation and is at least equivalent for PVI in patients with paroxysmal and persistent AF.1
Cryoablation in Atrial Fibrillation
Cryoablation is a safe and effective approach for the treatment of AF with a high rate of durable PVI and favourable mid-to-long term freedom from AF.2–10 It has a single procedure one-year success rate in paroxysmal AF of >80 % in patients no longer taking antiarrhythmic drugs.2–10 A large multicentre ablation registry study from Germany showed that the overall rates of major complications were similar in cryoablation and RF.11 We compared the results of 3,000 RF and balloon PVI procedures for AF and found a significantly reduced risk of cardiac tamponade in balloon ablation; no cardiac tamponade was observed in the cryoablation group.12 Different CB ablation techniques have been introduced – particularly for inferior pulmonary veins (PVs) – that aim to achieve acute PVI.3 These techniques are increasingly being utilised in different centres.
The recent multicentre randomised Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation (FIRE AND ICE) trial aimed to determine whether CB ablation was non-inferior to RF ablation in symptomatic patients with drug-refractory paroxysmal AF. After a mean 1.5 years of follow-up, the CB and RF groups had similar success rates and safety profiles. The results confirmed the comparable role of CB in PVI as an alternative to conventional RF ablation for paroxysmal AF.13 In a previous study with long-term follow up, success rate of 61.6 % was reported at median follow-up of 30 months in a group of 605 AF patients (579 with paroxysmal AF) who had undergone a single cryoablation procedure and were off antiarrhythmic drugs.14
Preparing the Patient for Ablation
In this review, we discuss current best practice and describe our centre´s procedural techniques using the CB ablation system for PVI, with a special emphasis on inferior PVs. The CB ablation system consists of a 15F steerable sheath (FlexCath™, Medtronic) and the CB, which is available in two different but fixed sizes (28 mm and 23 mm). The console delivers liquid N2O into the balloon. An intraluminal-mapping catheter (eight poles, 15 mm and 20 mm, Achieve™, Medtronic) serves as a guide wire along with PV mapping during CB ablation. The patient is asked to actively swallow an oesophageal temperature probe. Deep sedation is then induced using boluses of midazolam and a continuous infusion of propofol (1 %). Vital parameters, such as non-invasive blood pressure and oxygen saturation, are constantly monitored. For CB PVI procedures, we perform two right-sided venous punctures: one for the transseptal sheath and one to introduce a steerable diagnostic multipolar catheter that is subsequently positioned in the coronary sinus and superior vena cava for phrenic nerve (PN) stimulation.
Transseptal Puncture
Transseptal puncture is performed using a standard needle via a standard sheath. The transseptal needle is connected with a pressure monitor. We recommend a low and relatively anterior transseptal puncture as it offers greater mechanical advantages for the CB and Achieve mapping catheter when accessing PVs. Such a puncture allows more space for the CB to be rotated posteriorly to the right inferior PV (RIPV). A low puncture location can also improve balloon contact with inferior aspects of the inferior PV. Fluoroscopy and pressure monitoring are used to assist the transseptal puncture procedure. In difficult transseptal cases, use of transoesophageal echocardiography or intracardiac echocardiography is recommended.
Practical Techniques for Cryoballoon Ablation of Inferior Pulmonary Veins
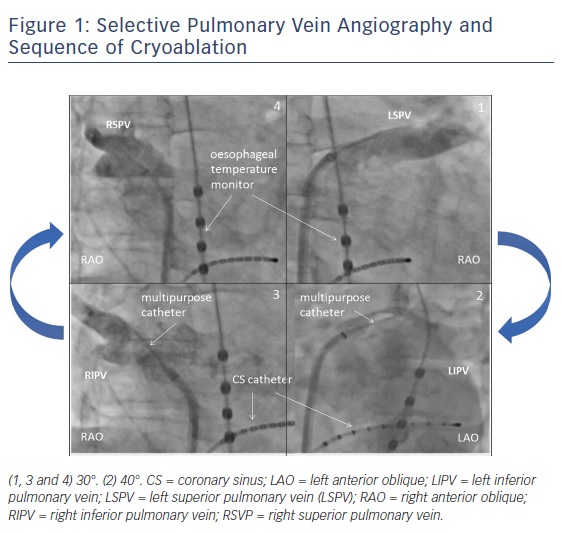
After a single transseptal puncture, selective PV angiographies are performed (using a 6F multipurpose catheter) to determine the PV and atrial anatomy. PV angiographies are performed using standardised angulations: right anterior oblique (RAO) 30° and left anterior oblique (LAO) 40°. Typically, the CB ablation follows a clockwise sequence: left superior PV (LSPV) then left inferior PV (LIPV), followed by RIPV and finally right superior PV (RSPV), see Figure 1. The rationale for this ablation sequence is discussed below.
Identification of LIPV and RIPV
Typically, inferior PVs are located posterior to superior PVs.15 Therefore, after LSPV ablation the steerable sheath is curved and rotated in a clockwise fashion in a posterior direction based on the baseline LIPV angiogram in left anterior oblique 40°. The intraluminal Achieve catheter should be advanced to intubate the LIPV while continuously analysing local electrical information. This mapping strategy can help safely navigate through the left atrium (LA).
After LIPV isolation, the CB should be pulled back into the sheath while the Achieve catheter remains in the LA and protecting the sheath. Again, clockwise rotation and curving of the sheath based on the RIPV angiogram (RAO 30°) should result in identification of the RIPV.
Ablation Techniques
In principle there are four different CB techniques that have been described: the direct approach, the hockey stick approach, pull-down and pull away.
Direct Approach
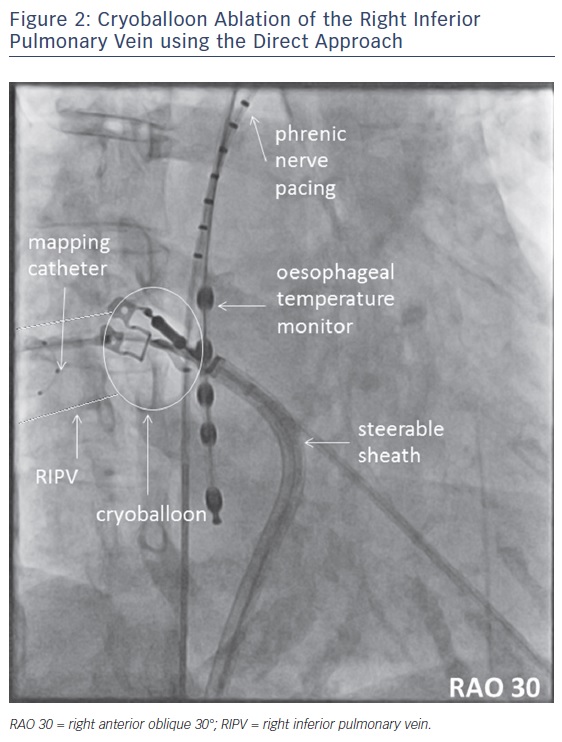
If the CB directly occludes the PV ostium, we use the term ‘direct approach’. This manoeuvre is typically used for LSPV and RSPV. It may be considered for selected RIPVs, see Figure 2. However, based on our experience this approach should not be the first choice for inferior PVs.
Hockey Stick and Pull-down
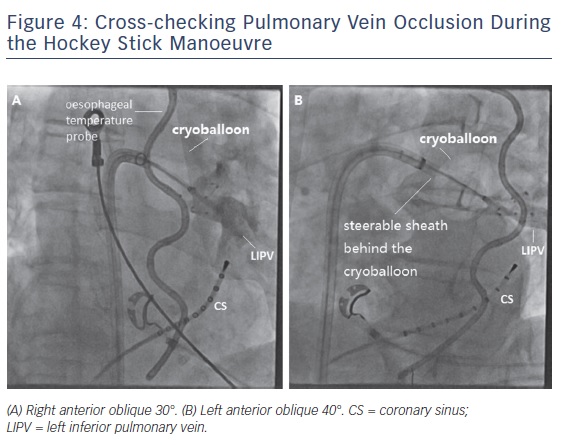
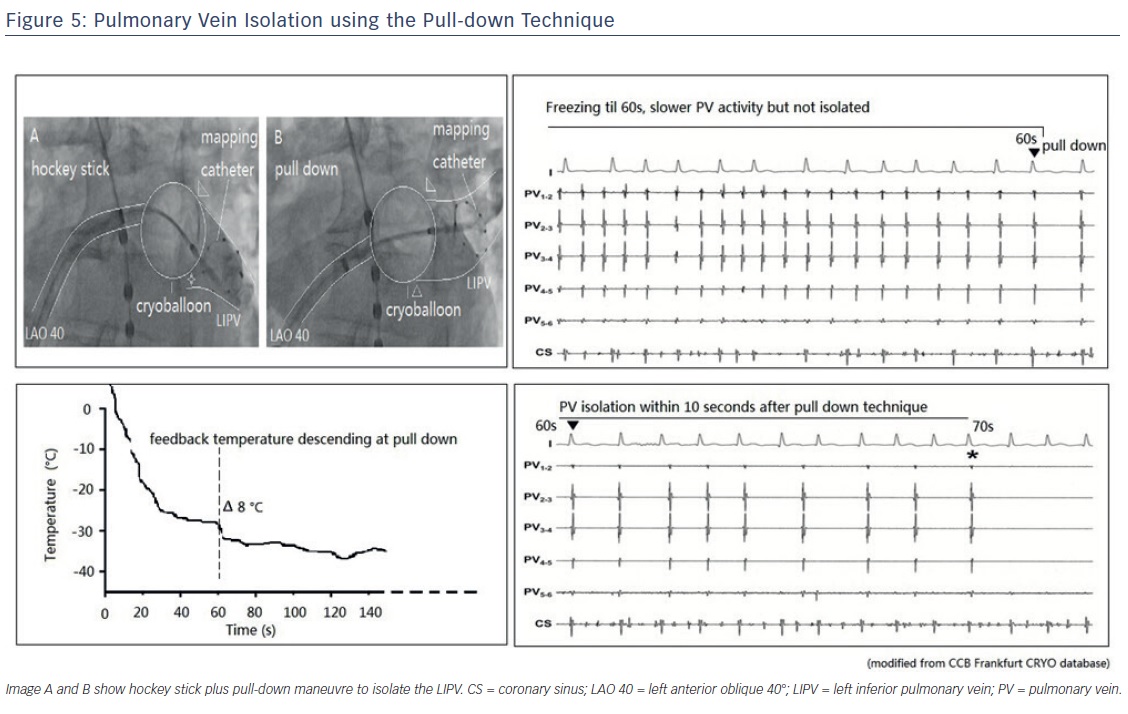
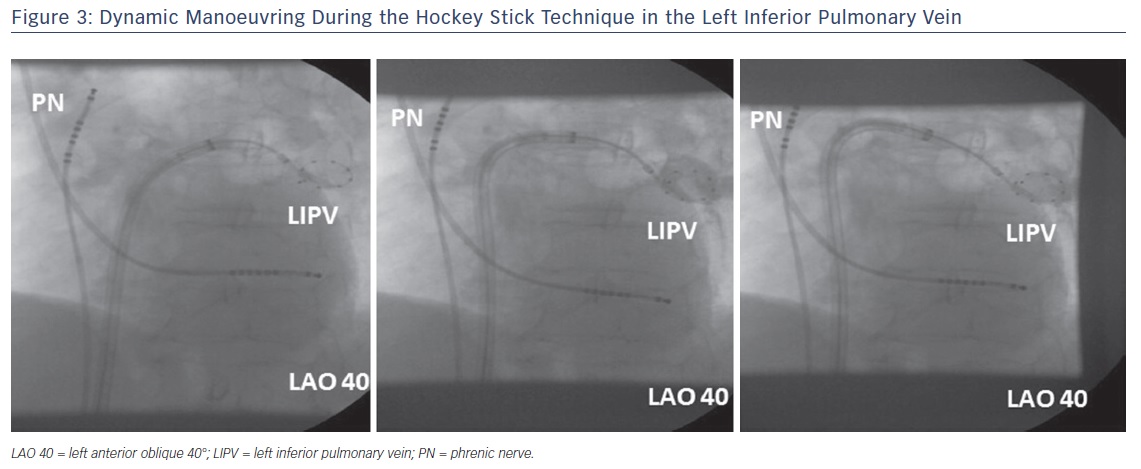
The so-called hockey stick alone or in combination with a pull-down manoeuvre is commonly used in LIPV and RIPV. A careful PV angiogram is required and the most caudal branch of the inferior PV should be wired with the spiral mapping catheter. After CB inflation, the sheath should be curved down and pushed up, with the bending point at the LA roof. The CB should then be advanced to improve contact with the inferior aspect of the inferior PV, resulting in a hockey stick figure on fluoroscopy, see Figure 3. Importantly, the CB should not slide into a distal PV position. The orientation of the steerable sheath should be aligned with the course of the spiral catheter; this may require a second look at the corresponding fluoroscopic angulation, see Figure 4. We also advance the steerable sheath to the proximal part of the CB to minimise potential CB and sheath dislocation, see Figure 4B. After optimal CB positioning, we carefully flip back the spiral catheter to increase PV signal visualisation without compromising the CB position.
If an inferior gap remains, we combine the hockey stick with a pull-down manoeuvre (CB and sheath) after 60 seconds. At this point in time, the CB is frozen to the superior aspect of the inferior PV and a typical response consists of an additional CB temperature drop. During freezing, the CB temperature profile is carefully monitored. If such a sequential PV occlusion has been achieved by the pull-down manoeuvre, PVI is expected to occur within the next 20 seconds, see Figure 5. However, if neither a temperature drop nor PVI occurs, freezing should be terminated and a different CB position obtained. The pull-down technique can also be combined with the direct approach for superior and inferior PVs.
Pull Away
The so-called pull away manoeuvre should be considered whenever the operator suspects an increased risk of PN palsy due to a short distance between the CB and the PN.16 Typically, during the initial phase of a freeze the CB is pushed towards the PV ostium. This may result in a distorted LA anatomy, thereby reducing the distance between the CB and the PN. Therefore, during CB freezing of RIPV or RSPV, the CB may be pulled away after about 60 seconds based on the idea of increasing the distance between the CB and the potential course of the PN.
Optimal CB Ablation/Dosing
Parameters that may be associated with effective CB lesions include PV occlusion, CB temperature profile, time to PVI and duration of freezing. CB freezing times can vary from 2 to 5 minutes per freezing cycle. A standard freeze duration of 4 minutes was utilised in the Clinical Study of the Arctic Front Cryoablation Balloon for the Treatment of Paroxysmal Atrial Fibrillation (STOP-AF) using a first-generation CB.17 In the FIRE AND ICE trial, an empiric 4-minute bonus freeze was applied to all PVs after acute PVI. Since the advent of the second-generation CB, a reduction in the duration of freezing to 3 minutes has been suggested. Investigators reported an 80 % success rate with 3 minutes of freezing in 143 patients in a single arm, non-controlled study.9
In the prospective randomised Individualised Cryoballoon Energy Pulmonary Vein Isolation Guided by Real-time Pulmonary Vein Recordings (ICE-T) trial, which included 100 patients with paroxysmal AF, we studied the impact of time to PVI where the duration of freezing was 4 minutes. If the time to PVI was <75 seconds, patients did not receive an empiric bonus freeze. Interestingly, the ICE-T protocol was associated with significantly reduced CB ablations while preserving the favourable rhythm outcome after 12 months.18 Procedure-related complications were numerically reduced in the ICE-T arm. Therefore, the ICE-T concept (time to PVI <75 seconds during 4-minute freeze with no empiric bonus) has been adopted as our institutional standard for CB AF ablation.
Real-time Pulmonary Vein Isolation
Recently, Wei and colleagues assessed real-time PV potential recordings during cryoablation in 180 AF patients. Real-time assessment of PV disconnection was achieved in 85.9 % of PVs.19 The third-generation CB with a short tip has been developed to improve the visualisation of real-time PVI recording; the rate of real-time PVI recording during cryoablation ranges from 74 % to 89.2 % in third-generation CB studies.20–23 In the ICE-T trial using second-generation CBs, real-time PVI was recorded in 80 % of PVs.18
Safety
Phrenic Nerve Function
Due to the anatomical proximity of the PN to the PVs (particularly the RSPV), CB ablation in the respective PVs can potentially lead to collateral PN injury. The incidence of transient right PN palsy as a complication of CB ablation of AF can reach around 20 % but persistent PN palsy remains uncommon, with a reported incidence 0–4 %.4-7,14 Most recently, Okishige and colleagues assessed left PN injury during cryoablation of the left PVs and reported transient and persistent left PN palsy rates of 6.5 % and 0.2 %, respectively.24
Before freezing, particularly for RSPV and RIPV, the PN should be paced at twice the capture threshold using a deflectable catheter to monitor PN function. An optimal site for right PN capture is near the junction of the superior vena cava (SVC) and the right subclavian vein or the anterolateral portion of the SVC near the atrial–SVC junction. For left PN capture, the optimal pacing site is the left subclavian vein with a pacing output exceeding the threshold by 10–20 %.24
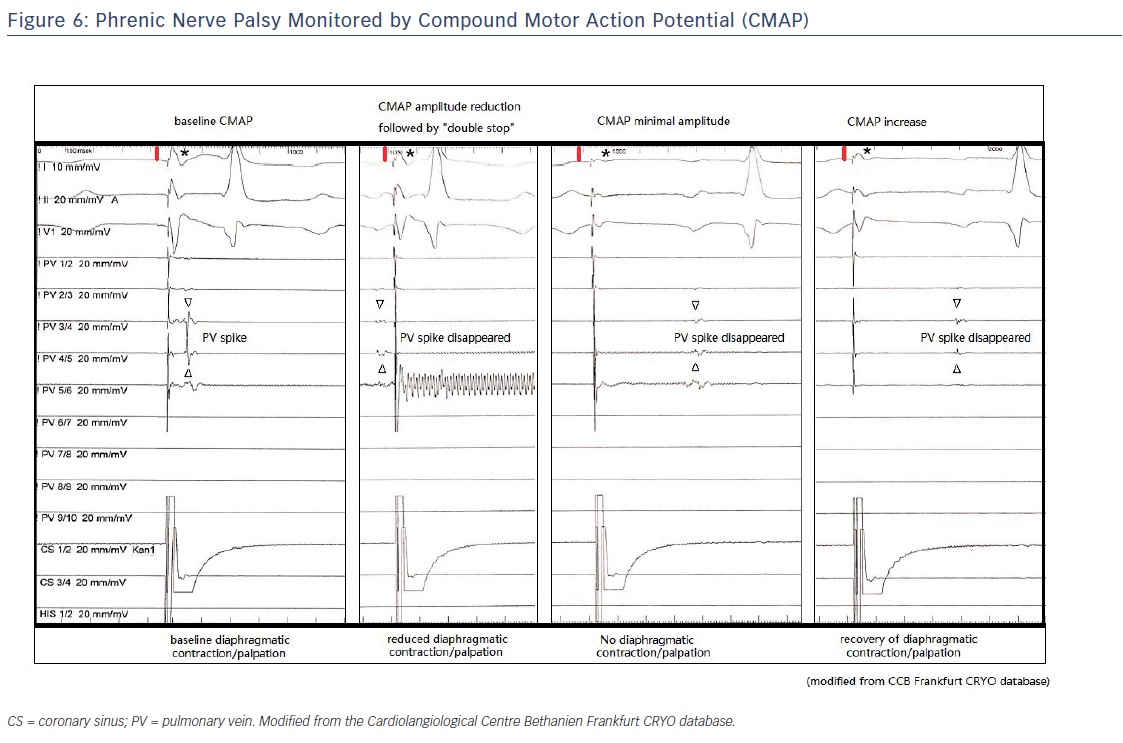
PN function can be monitored via manual palpation to determine the strength of the diaphragmatic excursion during PN pacing or by monitoring the diaphragmatic compound motor action potential (CMAP) during PN pacing (sensitive, early detection). One study reported that combining CMAP and palpation can decrease the incidence of PN palsy to <1.5 %.25
The concept of CMAP-guided ablation is relatively new. It has been demonstrated that a reduction in CMAP amplitude precedes diaphragmatic dysfunction.26 We recommend carefully monitoring both CMAP potential amplitude and diaphragmatic excursion (manual palpation). Observing CMAP has the added benefit that co-workers from the control room can assess the diaphragmatic muscle potential independently from the operator. An example of CMAP monitoring during cryoablation is shown in Figure 6. The CMAP is implemented using a modified ECG lead I technique. The right-arm surface ECG electrode is placed 5 cm above the xiphoid, and the left-arm surface ECG electrode is placed 16 cm from the xiphoid along the costal margin.
A recent study suggested that a larger PV dimension was associated with an increased risk of PN injury.27 Data from our group demonstrated that persistent PN palsy occurred in 2.8 % of patients undergoing first-generation CB and 1.9 % of patients undergoing second-generation CB ablation. First- and second-generation CB ablation resulted in transient PN injury (with full recovery before discharge) in 5.9 % and 3.8 % of patients, respectively. Complete recovery of phrenic function occurred after 29 + 11 days in first-generation CB ablation and 259 + 137 days in second-generation CB ablation.28
Ablation Sequence
We always perform CB ablation of the RIPV before the RSPV, see Figure 1. This sequence is based on the knowledge that CB RIPV ablation is associated with a lower risk of PN palsy as there is a greater distance to the PN. In addition, PVI typically precedes PN injury in the RSPV during CB ablation. Therefore, despite potential PN weakening at the RSPV, all four PVs can be isolated if the clockwise ablation sequence is selected.
Freezing Temperature
It is important to monitor the CB temperature during the procedure. The operator should understand and determine when to terminate a freeze cycle. The temperature displayed on the CryoConsole™ (Medtronic) is not the tissue temperature but a return gas temperature measurement. The balloon–tissue interface temperature is typically −70 to −80°C, whereas the temperature on the CryoConsole often ranges between −40 and −50°C.
A steep and rapid drop in temperature (<–40°C within 30 seconds) and nadir temperature of −55 to −65°C are potential indicators that the CB is in a deep PV position rather than an antral position. In this case, freezing should be terminated and the CB position re-confirmed.
For inferior PV, the nadir temperature achieved is normally between −35 and −45°C. Typically, an additional temperature drop can be observed after the pull-down manoeuvre.
Oesophageal Temperature
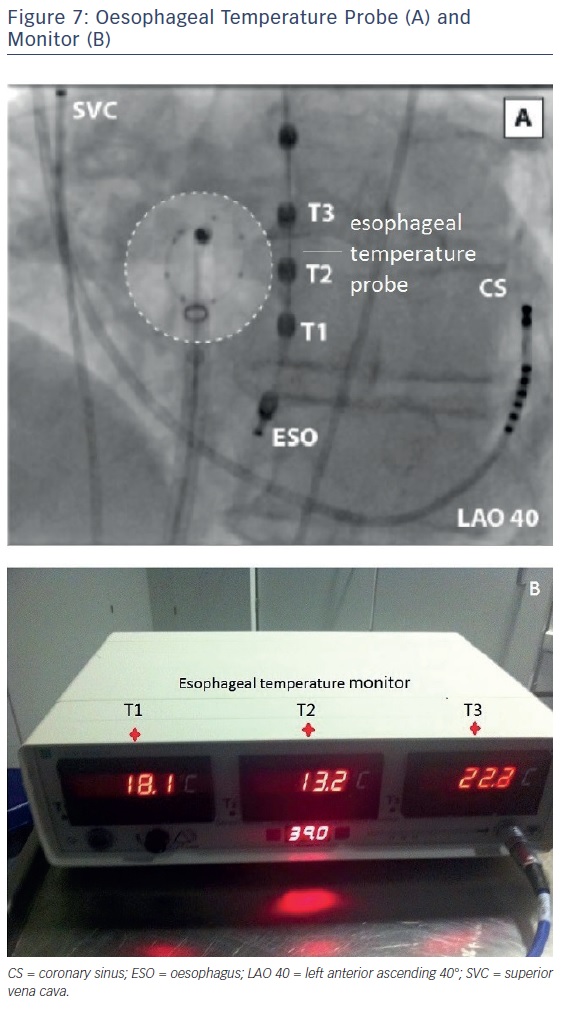
The temperature in the oesophagus should be constantly measured during CB ablation.29,30 The individual course of the oesophagus may not be predictable, but typically the region around the inferior PVs (LIPV) is the most common site for atrio-oesophageal fistula formation.29 A soft temperature probe, as shown in Figure 7, should be used with multiple thermocouples and a cut-off value of 15°C. This has been associated with a significantly reduced rate of post-ablation oesophageal lesions.30
Oesophageal Lesions and Atrio-oesophageal Fistulas
CB ablation can extended beyond the LA posterior wall and increase the potential risk of damaging neighbouring structures, such as the oesophagus. A 12–19 % incidence of oesophageal lesions after second-generation CB ablation and a correlation between lesions and the lowest endoluminal oesophageal temperature during freezing have been reported.31
Although most oesophageal lesions are asymptomatic and resolve after ablation, in rare cases oesophageal damage may result in a fatal atrio-oesophageal fistula. John and colleagues reviewed all documented cases of atrio-oesophageal fistula associated with CB ablation and found that it was most commonly related to the LIPV.29 Monitoring of the luminal oesophageal temperature is therefore highly recommended, particularly during CB ablation of inferior PVs. A recent study from our group reported that interruption of CB ablation based on endoluminal oesophageal temperature at a cut-off value of 15°C was associated with a very low (1.5 %) incidence of oesophageal injury.30 We recommend oesophageal temperature monitoring in CB cases and early termination of freezing whenever the oesophageal temperature drops to 15°C. We suggest that patients routinely receive 2 weeks of proton-pump inhibitors after cryoablation.
Fluoroscopy and Procedure Times
Mean fluoroscopy times reported from previous studies using second-generation CBs range from 13 to 29 minutes.7,32–34 In the FIRE AND ICE trial, the mean procedure time was shorter in the CB group than in the RF group (124 versus 141 minutes) and the mean total fluoroscopy time was shorter in the RF group than in the CB group (17 versus 22 minutes, p<0.001).13 In our recent ICE-T trial, the mean procedure time using the suggested techniques was 89±21 minutes and the fluoroscopy time was 12.7±5.5 minutes.18
PV Stenosis
Matsuda and colleagues evaluated the PVs of AF patients before and after cryoablation using cardiac-enhanced multidetector CT technology. The study found that PV stenosis occurred in 2.5 % of all the PVs analysed and all the stenoses were classified as being minimal (<25 %) or mild (25–50 %). The PV stenoses characterised by CT imaging did not progress further during the extended follow-up period.35
Narui and colleagues studied the PVs of patients who underwent CB ablation for paroxysmal AF using contrast-enhanced CT both before and 3 months after the procedure. There were reductions in the dimensions of PVs with mild (25–50 %), moderate (50–75 %) and severe (≥75 %) values in 29.3 %, 4.7 %, and 1 % of the PVs analysed, respectively; a larger PV ostium and lower minimum freezing temperature during CB ablation were independently associated with PV narrowing.36
Haemoptysis and Lung Injury
Haemoptysis has been reported following cryoablation of AF; however, it remains uncommon, with an incidence ranging between 0 and 2 %.37–39 Transient interruption of vascular integrity, perhaps within the pulmonary capillary system due to cryoinjury, has been postulated as the reason for haemoptysis. Erosion can also be detected during bronchoscopy.37–39 Based on the evidence, both the clinical symptoms and findings appear to be self-limiting, with gradual resolution over time. No evidence has shown that these cases have been associated with catastrophic complications, such as the formation of a fistula.
Advantages and Limitations
Advantages of cryoablation for AF include: a homogenous and extended lesion; it is less arrhythmogenic; it enables real-time PV isolation without the need for a 3D mapping system; and it reduces the procedure time. Currently, cryoablation is limited to instances of non-PV trigger, additional substrate modification and special PV anatomies.
A recent study reported using a CB catheter to achieve large-area atrial substrate modification in persistent and long-standing persistent AF. The success rate at 12 months was 71 % in patients with persistent AF and 55 % in patients with long-standing persistent AF.40 Akkaya and colleagues reported a 70.3 % success rate at 37 months in patients who had undergone PVI plus left atrial roof-line ablation using a CB.41
Patients with a left common pulmonary vein (LCPV) can be a challenge for PVI using CB technology due to the size of the CB. Antral level isolation of the LCPV ostium was achieved in half of the patients within the LCPV group in a recent trial; in patients where antral PVI could not be obtained, sequential isolation of the first superior and inferior PV branches was applied. The follow-up data showed comparable results with regard to clinical success and the durability of PVI in patients with or without LCPV.42
Summary
CB PV isolation has been established in AF ablation. This review focuses on key procedural aspects and illustrates practical techniques in CB PVI to shorten the learning curve without compromising safety and efficacy, with special emphasis on inferior PVs. Specific CB manoeuvres and safety measures should be adopted for inferior PVs, thereby facilitating the endpoint of PVI. It should be mentioned that the techniques suggested in this paper are based on the procedural experience of a high-volume centre and further studies are warranted.