Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia observed in clinical practice, occurring in approximately 2 % of the general population.1–3 A progressive increase in both the prevalence and incidence of AF has been demonstrated in recent years, defining AF as a major economic and public health issue.1
The identification of sites of AF initiation and/or maintenance within the pulmonary veins (PVs) has led to the development of percutaneous procedures to electrically isolate the PVs from the left atrium (LA).4 Large observational studies and multiple randomised-controlled trials have demonstrated that catheter ablation is universally superior to anti-arrhythmic drugs (AADs) for the maintenance of sinus rhythm (66–89 % versus 9–58 %, respectively) and results in a greater improvement in arrhythmia-related symptoms, exercise capacity and quality of life.1,2,5–8 As a result, catheter ablation has become the ‘standard of care’ for the maintenance of sinus rhythm in symptomatic patients in whom drugs are ineffective or poorly tolerated.
While the results of ablation are unequivocally superior to medical therapy, they are unfortunately not flawless: approximately 30 % of paroxysmal AF patients will experience arrhythmia recurrence after a single ablation procedure.8 As most recurrences are in association with PV reconnection or as a result of non-PV triggers, several pharmacological challenges have been proposed to improve outcomes.9–12 This article reviews the pathophysiological background and evidence for the use of pharmacological challenges during catheter ablation procedures.
Pathophysiology of Atrial Fibrillation and Atrial Fibrillation Catheter Ablation
Despite decades of progress, there is no comprehensive pathophysiological explanation of AF. Early hypotheses postulated that AF resulted from the co-existence of multiple independent wavelets propagating randomly throughout the left and right atria (the ‘multiple wavelet hypothesis’).13,14 This hypothesis suggested that as long as the atria had a sufficient electrical mass, and an adequately short refractory period, AF could be initiated and indefinitely perpetuated.15 Based on this theory, the early surgical interventions for AF were designed to reduce the excitable mass of atrial tissue by compartmentalising the atria into smaller regions incapable of sustaining a critical number of circulating wavelets.16 Unfortunately this strategy has proved to be of limited efficacy and has been associated with a substantial risk of major complications.17
In the late 1990s, Haïssaguerre and colleagues demonstrated that AF is a triggered arrhythmia initiated by rapid repetitive discharges, predominantly from the proximal aspect of the PVs.4 This discovery led to the development of percutaneous procedures to directly eliminate spontaneous focal ectopic activity within the PVs. However, early AF recurrences from the targeted and other nontargeted PVs led to modification of the ablation strategy to electrically isolate all of the PVs.18,19 Over the past 17 years, the recognition that sites of AF initiation and/or maintenance (e.g. triggered activity and micro re-entry) are frequently located within the PV antrum has shifted the ablation target more proximally.20–22 As such, the contemporary AF ablation procedure is a hybrid approach whereby circumferential ablative lesions are placed within the peri-venous left atrial myocardium, i.e. outside of the tubular veins with the goal of electrical pulmonary vein isolation (PVI). Successful electrical PVI is defined as a bidirectional conduction block documented using a circular mapping catheter placed at the PV ostia. Ablation is able to target both the initiating triggers, as well as the mass of electrically-active LA tissue capable of sustaining the fibrillatory wavelets responsible for AF perpetuation.3 It also has the advantage of limiting PV stenosis.23
While isolation of the PV antra has become the cornerstone of all contemporary AF ablation procedures, patients with more advanced forms of AF, e.g. persistent rather than paroxysmal AF, are known to be less dependent on the PV antra for arrhythmia initiation and perpetuation.24–26 As the disease progresses, electrical and structural remodelling of the atrial substrate shifts the sites of AF perpetuation to regions outside the PV–LA junction and results in the emergence of non-PV triggers.27–29 The ablation of these ‘fibrotic atrial’ forms of AF often requires adjunctive strategies targeting the abnormal LA substrate, such as linear LA ablation with the goal of compartmentalising the LA into smaller regions incapable of sustaining micro re-entry, or the ablation of complex fractionated atrial electrograms (CFAEs, or areas of abnormal substrate representing areas of slow conduction, conduction block or local ‘pivot’ points) that perpetuate AF re-entry.20,30–33 However, the addition of such substrate-based ablation (either linear ablation or CFAE elimination) does not appear to reduce AF recurrence after PVI in patients with persistent AF.34
It has been suggested that ganglionated plexi may have a role in the initiation and maintenance of both paroxysmal and non-paroxysmal AF.35–41 Localisation is usually performed on the endocardium either anatomically, by vagal response following high-frequency stimulation, or by Fourier transform in sinus rhythm.35,37 Although ganglionated plexi ablation significantly reduces AF recurrence, the long-term success rate is lower than after PVI.35–41 Interestingly, in addition to PVI, the suppression of ganglionated plexi response – particularly that observed during cryoablation – may reduce AF recurrence.40,41
Although many authors believe that additional ablations are required for non-paroxysmal AF or some paroxysmal AF, no randomised studies have consistently shown which strategy to use.
Mechanism of Atrial Fibrillation Recurrence After Ablation
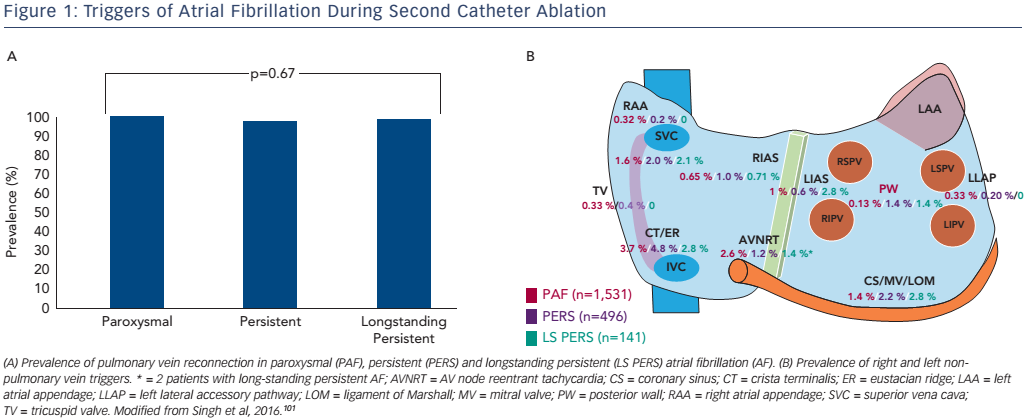
Unfortunately, the results of ablation can be unsatisfactory. In the case of paroxysmal AF, only about 70 % of patients will remain arrhythmiafree after a single ablation procedure without the use of AADs.1,2,5–8 It is important to recognise that the reasons for long-term failure are largely centred on the relative inability to create a lasting transmural lesion using the contemporary ablation toolset. While electrical PVI may be achieved acutely, the combination of inadequate electrode– tissue contact, insufficient power delivery and tissue oedema may prevent radiofrequency (RF)-induced heating of the myocardium to lethal temperatures. 27,42–44 As the transient injury induced at the time of index ablation resolves, gaps in the initial line of ablation may emerge, allowing PV triggers to excite the adjacent LA and induce AF.27 This is highlighted by the observation that >90 % of patients requiring a second catheter ablation procedure demonstrate one (or more) PV reconnections (see Figure 1A).26,45
For patients with more advanced forms of AF, recurrences may be due to the persistence of LA substrate abnormality as well as non-PV triggers (see Figure 1B). These triggers can be found in about 50 % of patients and originate in the superior vena cava, left atrial free wall or appendage, coronary sinus, crista terminalis or right atrial free wall.27 Targeted ablation of these non-PV triggers has been shown to improve outcomes, but unfortunately is limited by non-inducibility at the time of the ablation procedure, as well as unreliable long-term behaviour over time.9,33,46
Pharmacological Challenges in Catheter Ablation of Atrial Fibrillation
Four pharmacological adjuncts have been proposed to improve the outcomes of AF ablation. These four agents – isoproterenol, adenosine, amiodarone and ibutilide – are mechanistically disparate and are used for different purposes: unmasking dormant conduction (DC), inducing non-PV triggers or identifying abnormal substrate for ablation.
Adenosine
Adenosine is predominantly used to differentiate permanent PV-atrial conduction block from DC (i.e. viable but latently non-conducting tissue).
Mechanism of Action
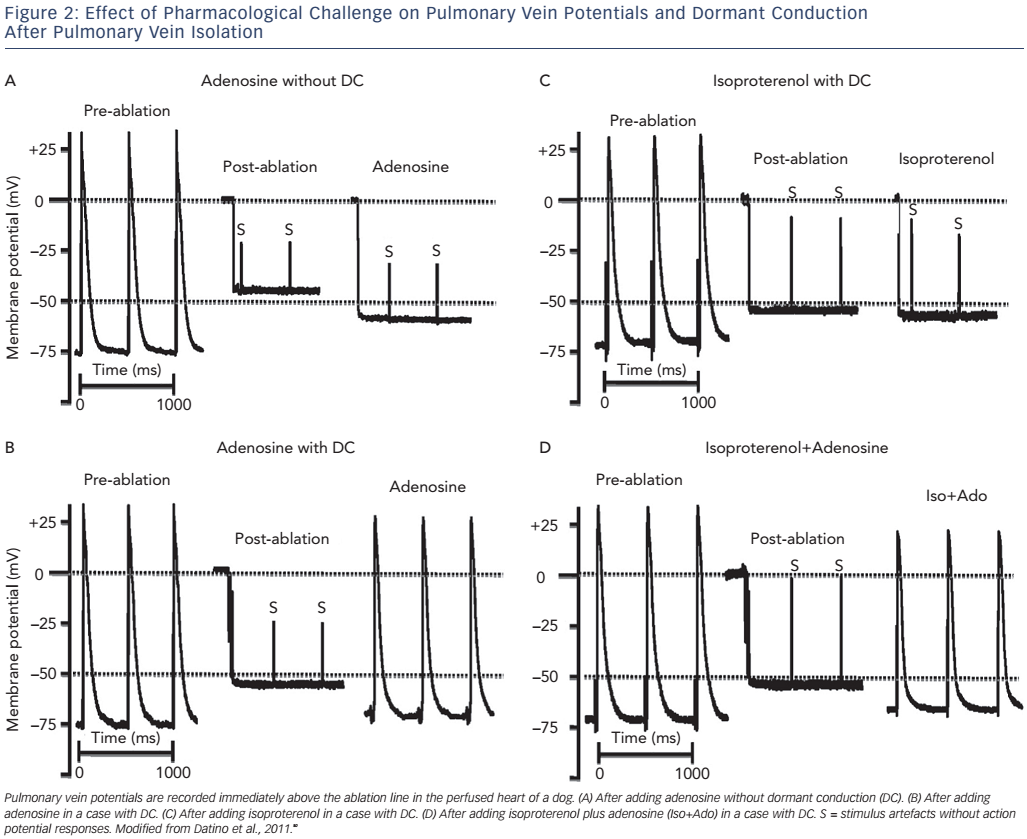
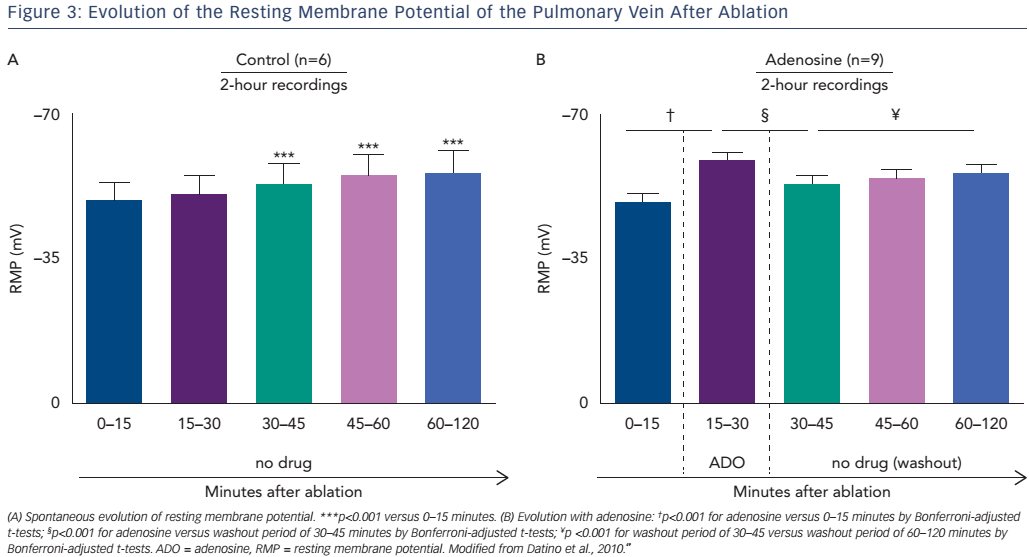
Following ablation, the resting membrane potential (RMP) of the targeted left atrial myocardial cells becomes depolarised due to cell membrane injury. This depolarisation of the RMP results in sodiumchannel inactivation (when >−60mV), leading to inexcitability and functional conduction block.47,48 After a waiting period of 30–60 minutes, a slow hyperpolarisation can be observed leading to spontaneous PV reconnection, called DC.49 The difference between dormant and nondormant PVs lies primarily in the degree of RF-induced depolarisation. Non-dormant PVs are depolarised more severely (post-ablation RMPs positive to −50 mV) than dormant PVs (post-ablation RMPs of −50 to −60 mV).49
Adenosine has been proposed as a useful test of DC due to its differential effect on PV cells and LA cells.50 In both the PV and LA cells adenosine is able to shorten the action potential duration, however it selectively hyperpolarises the RMP by about 10 mV and increases dV/dt (max) by selectively activating IKAdo in PV cells (leading to an increase in the transient outward potassium currents; see Figure 1).49,51,52 Moreover adenosine’s effect on the PV sodium channel removes voltage-dependent INa inactivation, and further increases the dV/dt (maximum velocity of phase 0 of the action potential; see Figure 2).49,51,52 Taken together, in the event of incomplete membrane damage after RF ablation, adenosine can facilitate membrane hyperpolarisation, restoring the excitability threshold see (Figure 1). Conversely, those cells that have sustained irreversible damage will not respond to adenosine infusion, i.e. the membrane will remain depolarised and unexcitable. Additionally, adenosine may reveal nonPV triggers secondary to post-bradycardia adrenergic simulation.9,49
Clinical Value
In 2004, Arentz et al. demonstrated that adenosine could be used to reveal DC.53 Subsequent observational studies have demonstrated DC in 25–51 % of cases after PVI using RF. These studies have suggested that ablation guided by adenosine triphosphate (ATP)/adenosine administration can reduce AF recurrences at 1 year by 32–50 % (relative risk reduction).53–57 Despite a lower incidence of DC (13–40 %), a similar effect has been suggested after cryoablation of the PV.58,59
The recently-published randomised Adenosine Following Pulmonary Vein Isolation to Target Dormant Conduction Elimination (ADVICE) study was the first to prospectively evaluate the impact of adenosine testing on clinical outcomes after AF ablation.60 After PV isolation using an irrigated-tip RF catheter, adenosine revealed DC in 53 % of patients. Those with DC were randomised to additional adenosineguided RF ablation until DC was eliminated or no further ablation. In this study a 56 % relative-risk reduction (27 % absolute risk reduction) in the recurrence of atrial tachyarrhythmias was observed with the elimination of DC. In contrast, the Unmasking Dormant Electrical Reconduction by Adenosine Triphosphate (UNDER-ATP) study and the study by Ghanbari et al. failed to demonstrate a significant difference in the reduction of AF recurrence between adenosine-guided PVI and conventional PVI (1-year event-free survival of 68.7 % with ATP-guided versus 67.1 % without ATP, p=0.25 in UNDER-ATP; and 61 % with adenosine plus isoproterenol versus 66 % with isoproterenol alone, p=0.83 in Ghanbari et al.).61,62
Differences in the studies’ methodology and approach may explain these results. First, the endpoint of adenosine testing in the ADVICE study and Ghanbari et al. was based on titration of the adenosine dose until the intended electrophysiological effect (transient AV block or sinus arrest) was observed. Conversely, in the UNDER-ATP study the dose of adenosine was predetermined (0.4 mg/kg) and was not altered regardless of the observed effect. Given the lack of documentation of adenosine effect, it is possible that patients in the UNDER-ATP study were underdosed. Second, the waiting period between the achievement of index PVI and adenosine test varied between the studies. In the ADVICE study it was 20 minutes after isolation of the last PV, while in Ghanbari et al. it was 60 minutes, and in UNDER-ATP there was no specific protocol regarding the timing of adenosine administration. In effect this resulted in a median waiting period in UNDER-ATP and Ghanbari et al. that was more than double that of the ADVICE trial. This is relevant given the knowledge that spontaneous recovery of PV–LA conduction is a time-dependent process, with spontaneous RMP hyperpolarisation occurring approximately 30 minutes after ablation.49,50 Mechanistically the administration of adenosine results in a more rapid hyperpolarisation, effectively predicting the spontaneous reconnections that occur between 20 and 60 minutes post-PVI (Figure 3).60,63–66 Taken together it is not surprising that the UNDER-ATP trial and Ghanbari et al. had a higher rate of spontaneous PV reconnection (42.6 % in UNDER-ATP versus 27 % in ADVICE) and a lower rate of DC (27.6 % versus 53 % in the ADVICE study and 37 % in the Ghanbari et al study).53–57,60 Third, as a result of the low prevalence of DC the UNDER-ATP trial was underpowered. Lastly, The ADVICE study only included patients with paroxysmal AF treated with PVI alone, while the UNDER-ATP study included patients with persistent AF (32.8 %) treated with PVI accompanied by additional linear lesions or complex electrogram ablation. While adenosine testing lacks substantial effect in the case of additional linear lesions or the ablation of non-PV triggers, the delivery of additional substrateguided ablation (roof line, mitral isthmus line, superior vena cava isolation and CFAE elimination) would have conferred an even longer waiting period. Thus the pathophysiology of persistent AF, the longer post-PVI waiting period and the relative underdosing of adenosine could all explain the low rate of DC revealed with adenosine testing in the UNDER-ATP study. As such, we can conclude the use of adenosine testing for DC is useful in paroxysmal AF patients when an adequate adenosine dose (titrated to clinical effect) is administered after a fixed waiting period (20 minutes).
Concomitant use of dipyridamole has been suggested to prolong the transient effect of adenosine in DC and to reduce AF recurrence by facilitating the elimination of DC.67,68 The global outcome of such a strategy, however, remains to be assessed.
Isoproterenol
Isoproterenol is used predominantly to identify non-PV triggers that have been associated with AF recurrence, particularly in those with persistent AF.69–74 These triggers may originate from the superior vena cava, coronary sinus, interatrial septum, crista terminalis, Eustachian ridge, inferior mitral annulus, atrial appendages, persistent left superior vena cava and ligament of Marshall. When present, nonPV triggers have been associated with AF recurrence and a worse outcome after ablation.69–74 Fortunately these sites can be revealed in patients with paroxysmal and persistent AF with the infusion of highdose isoproterenol. 9,11,75
Mechanism of Action
Isoproterenol is a cardiac beta1 and beta2 adrenoreceptor agonist with positive chronotropic, dromotropic and inotropic effects. Via the cyclic adenosine monophosphate mechanism, isoproterenol results in an increase in diastolic [Ca2+] i and intracellular Ca2+, decreasing the action potential duration and atrial refractory periods while facilitating slow diastolic depolarisation (abnormal automaticity) and triggered activity.76–79 The ability to reveal triggered activity is significantly greater with isoproterenol than adenosine.9,49
With respect to DC, isoproterenol induces a mild hyperpolarisation due to its summative effect on K+ currents (particularly the inward rectifier current, or IK1), cyclic adenosine monophosphate-activated Cl − currents and the pacemaker current, If, as well as its secondary effects on L-type Ca2+ currents and the Na+–Ca 2+ exchanger.80 The magnitude of the effect on the RMP is minimal, i.e. no different from controls, however, being significantly smaller than that observed with adenosine, and is insufficient to restore conduction in dormant veins (see Figure 2).49,50
Clinical Value
Observational studies have suggested that PVI accompanied by the ablation of non-PV triggers unmasked by isoproterenol infusion could improve the success rate of catheter ablation.69–74,81–83 Non-PV triggerablation protocols generally involve burst-pacing protocols with isoproterenol infusion to induce the triggers, followed by mapping and elimination. Unfortunately the utility of non-PV trigger elimination is limited by the difficulty in inducing, identifying and eliminating these non-PV triggers. In different cohorts the prevalence of non-PV triggers varies between 9 and 19 % (9 % in Inoue et al.’s study of 263 persistent AF patients, 11 % in Santangelli et al.’s study of 2,168 patients with paroxysmal and persistent AF, and 19 % in Lin et al.’s study of 130 patients with long-standing persistent AF).33,84,85 This incidence seems to increase with age, worse atrial substrate and in the presence of cardiomyopathy.81,86 Despite the identification of non-PV trigger sites, however, only 30 % of these can be eliminated due to difficulties in localising them.85 That said, a better arrhythmia-free outcome has been observed in patients in whom all PV and non-PV triggers are eliminated when compared with those in whom triggers are identified but cannot be eliminated (86 % versus 37 %; p=0.09).85
It is not clear whether current protocols are able to reliably identify all relevant non-PV trigger sites. As such, it has been postulated that empiric ablation of common non-PV trigger sites may improve outcomes. This has been examined in the Randomized Ablation Strategies for the Treatment of Persistent Atrial Fibrillation (RASTA) study, which compares: circumferential PVI plus ablation of nonPV triggers; circumferential PVI plus ablation of non-PV triggers plus empirical ablation at common non-PV trigger sites; and circumferential PVI plus ablation of non-PV triggers plus CFAE ablation.87 The freedom from atrial arrhythmias after a single ablation procedure was significantly worse with the addition of CFAE (29 %) when compared with PVI plus non-PV triggers alone (49 %, p<0.040) and PVI plus non-PV triggers plus empirical trigger-site ablation (58 %, p<0.004).
Clinical Perspective
- Adenosine can prevent the need for a long observational time to identify dormant conduction that will increase the recurrence of AF after pulmonary vein isolation (PVI).
- The role of adenosine in a second ablation procedure for the treatment of paroxysmal AF and in the catheter ablation of persistent AF remains to be assessed.
- In patients with paroxysmal and persistent AF, the use of isoproterenol should be considered in appropriate cases as its ability to reveal non-PV triggers has been demonstrated.
- Amiodarone and ibutilide allow the organisation of electrical substrates in persistent AF but they do not appear to be efficient in reducing AF recurrence.
Last, the relevance of targeting non-PV triggers remains a matter of debate. Most studies considered repetitive premature atrial contraction as the target for ablation.9,33, 81–87 Several repetitive premature atrial contractions, however, will never induce AF. As such, an increased success rate has been achieved when targeting only the premature atrial contractions that induce AF.9 Thus, non-PV AF trigger could explain the variation observed in the prevalence and outcomes in different groups.
Amiodarone and ibutilide
Amiodarone and ibutilide are used to organise persistent AF.
Mechanism of Action
In advanced forms of AF, the abnormal atrial substrate is thought to act as a driver of arrhythmia perpetuation.88,89 Although PVI can reduce the amount of substrate required for atrial re-entry, persistent AF seems to be less dependent on the PV antral region for arrhythmia initiation and perpetuation, relying more on perpetuating regions outside the PV–LA junction. It has been postulated that these CFAEs (local signals during AF that are either at a very short cycle length, or are fractionated with two or more components and/or a continuous perturbation of the baseline) represent areas of slow conduction, conduction block or ‘pivot’ points for AF perpetuating re-entry. It is thought that complete elimination of these abnormal substrate areas may improve outcomes.90–92 Extensive ablation of atrial substrate may result in prolonged procedures, however, and increased risk of complications.93,94 Moreover, while fractionation may be recorded close to the core of an AF-perpetuating rotor, it may also be recorded at sites not actively participating in the AF process, i.e. bystander sites of passive wavelet collision. It is postulated that the co-administration of amiodarone and ibutilide (both class III AADs) might facilitate the identification of CFAE sites critical to AF maintenance by eliminating areas of passive atrial activation. Mechanistically these agents differentiate active from passive CFAEs by lengthening the effective refractory period (e.g. global AF cycle length). Pre-treatment with amiodarone or the administration of ibutilide during catheter ablation to reduce active CFAE sites has been shown to reduce the amount of ablation in persistent AF without adversely affecting longerterm outcomes.95,96
Clinical Value
The Substrate Trigger Ablation for Reduction of Atrial Fibrillation II (STAR-AF II) study recently demonstrated that the addition of substrate-base ablation (either linear ablation or CFAE elimination) did not reduce AF recurrence after PVI in patients with persistent AF.34 These negative results could be explained by the amount of ablation that increases the iatrogenic arrhythmia rate.87,97–99 To reduce this arrhythmia rate, two recent randomised studies have investigated the effect of ibutilide and amiodarone with respect to PVI and CFAE ablation. Mohanty et al. described a 112-patient population treated with amiodarone for persistent AF.100 Patients were randomised to either amiodarone continuation or amiodarone discontinuation 4 months prior to catheter ablation. The authors observed a higher organisation rate of AF, and a lower amount of RF energy required terminate AF in the amiodarone continuation group. Despite this, they observed an increase in AF recurrence with amiodarone continuation. In the Modified Ablation Guided by Ibutilide Use in Chronic Atrial Fibrillation (MAGIC-AF) study, Singh et al. randomly assigned 200 patients with persistent AF to receive ibutilide during catheter ablation.101 A higher AF organisation rate, a reduction in the number of CFAE sites and a higher rate of AF termination were observed during catheter ablation in the ibutilide group, similar to the study by Mohanty et al.101 Likewise the clinical outcomes were unchanged. These results once again suggest the limited role of substrate ablation instead of PVI.
Conclusion
PVI remains the cornerstone of catheter ablation for the treatment of both paroxysmal and persistent AF, but the durability of electrical isolation remains a challenge. Both longer observational time (>30 minutes) and adenosine testing (>20 minutes after successful PVI with dose titration to achieve transient atrioventricular conduction block) are efficient and should be considered after PVI to decrease long-term PV reconnection and AF recurrences in paroxysmal AF.
Isoproterenol may unmask non-PV triggers in appropriate cases. The benefit seems to be relatively greater during a second ablation procedure, particularly if one or more PVs have reconnected. Limited data are available to support the use of amiodarone and ibutilide; despite their role in substrate organisation, the lack of impact on clinical outcome does not suggest a relevant use in catheter ablation of AF.