Heart rate and blood pressure are tightly regulated by autonomic control to effect adequate blood flow as needed. This regulatory process breaks down when the vasovagal reflex is activated. Profound, but brief, circulatory collapse manifests as bradycardia (cardioinhibitory response) and/or hypotension (vasodepressor response) and/or altered cerebral autoregulation, resulting in transient loss of consciousness, often with prodromal signs and symptoms (pallor, sweating and nausea) and profound fatigue and nausea during recovery.1,2 This complex neurocardiogenic reflex presents with a wide range of clinical scenarios, from isolated, sporadic, easily explained episodes, to frequent, recurrent and baffling events that, while not usually life-threatening, can be devastating.3,4 Vasovagal syncope (VVS) in otherwise healthy individuals is the most common cause for syncope.5,6 The vasovagal reflex is often responsible for syncope in other conditions as well, such as pulmonary emboli, aortic stenosis, hypertrophic cardiomyopathy, inferior myocardial infarction, gastrointestinal bleeding and dehydration.
The exact mechanism underlying and triggering the vasovagal reflex continues to be studied and debated, but inputs causing the reflex are manifold.7 Afferent signals from the peripheral vagus (and perhaps from central locations), processed in the nucleus tractus solitarius, elicit an efferent, phasic, abrupt and rapidly reversible parasympathetic response, causing transient relative sinus bradycardia, sinus arrest or paroxysmal atrioventricular (AV) block.8. Frequently, there is sudden disappearance of muscle sympathetic nerve activity at a time of diminishing cardiac output, causing vasodilation and hypotension,9-11 and it has been argued the bradycardia and asystole are due to sympathetic withdrawal.12 There can be impairment of ventricular contractility, with consequent reduction in cardiac output,13,14 and changes in cerebrovascular autoregulation.15 There are influences of circulating mediators, such as epinephrine, renin, endothelin, vasopressin, cortisol, prolactin, beta-endorphins, substance P, nitric oxide synthase and even adenosine, in some individuals.16–19
One postulated initiating factor is transient, excess, time-dependent, sympathetic activation, causing increased ventricular contractility, subsequent mechanoreceptor activation, initiating vagal (atrial and/or ventricular C fibre) afferent activation that is epinephrine dependent and the subsequent reflex.20 However, for those who are susceptible, triggers include prolonged standing, pain, psychological or emotional stressors, noxious stimuli or, simply, nothing at all.21–23 The reflex may be manifest in many ways and no individual necessarily has a unique footprint of their physiological perturbations.
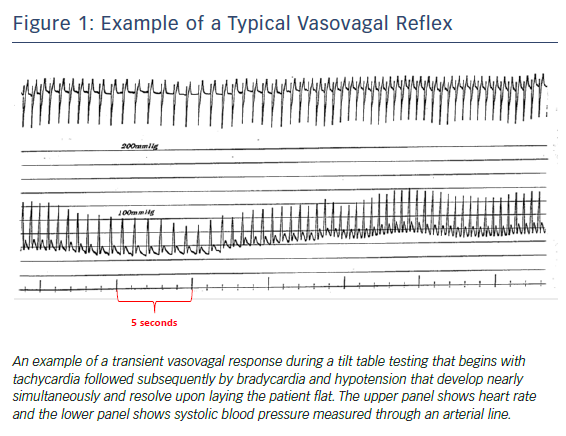
Two processes, parasympathetic activation and sympathetic inhibition, are linked but are not necessarily concomitant or the same for recurrent events.24 Tachycardia may precede bradycardia and collapse (Figure 1).25 Whatever the mechanism, it is complex, different in younger versus older individuals, occurs in phases (often with contradictory physiology) and resolves rapidly and mysteriously likely in concert with enhanced venous return.7
Preventing Recurrence of Vasovagal Syncope
While most episodes of VVS are self-limiting, for some, the problem can be recurrent and devastating. Multiple non-pharmacological approaches may modulate the vasovagal response. Adequate hydration, avoidance of triggering events (e.g. donating blood) and physical counter-pressure manoeuvres during an event have been advocated,26,27 yet, no robust data point to any benefit of these recommendations or any effective pharmacological approach. This is not surprising, since the reflex is abrupt and transient and so any tonic intervention would not necessarily prevent an occasional autonomic destabilisation and may in fact worsen episodes if the autonomic nervous system otherwise becomes unbalanced.28 Pacing has been postulated to be effective as it can prevent severe bradycardia and asystole. Here, we explore the data on the use of pacing to prevent recurrent VVS.
Caveats Concerning Vasovagal Syncope
A prodrome, often present in VVS, may occur before bradycardia or hypotension.29 It is likely that this involves early changes in the autonomic activation and cardiac contractility before the faint.12 The relationship of bradycardia to hypotension can vary from person to person, and between episodes in the same individual (Table 1).24 Furthermore, there is no physiological gold standard to assess VVS; tilt-testing (with or without adjunct nitroglycerin or isoproterenol) does not emulate what happens in real life and thus may not reproduce heart rate and blood pressure responses that occur spontaneously.30
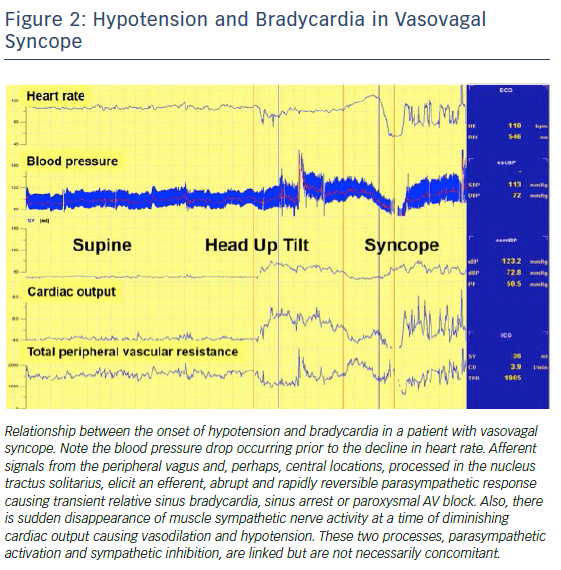
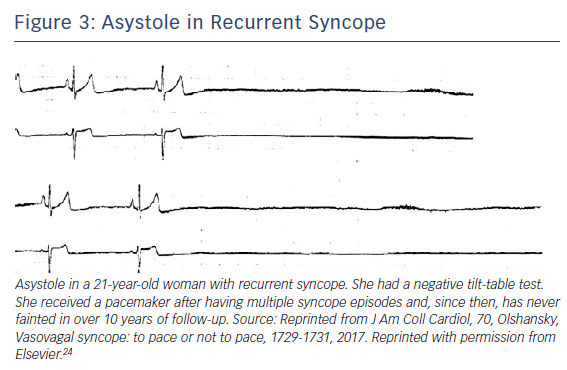
Recently, Saal et al. used video recordings, electroencephalography, and blood pressure and heart rate measurements to assess the relationship of bradycardia and hypotension to loss of consciousness during a vasovagal faint. Asystole occurred after the faint began in one-third of cases, making it unlikely to be the primary cause of syncope, and suggesting that bradycardia monitoring alone may overestimate which patients with VVS actually passed out from asystole31 (Figure 2). The incidence and extent of bradycardia, presumably related to a vasovagal reflex, but as a cause for syncope, may be age-dependent and confusing. While the incidence of bradycardia and asystole due to the vasovagal reflex may not vary by age as determined by tilt table testing,32,33 bradycardia in older patients may present due to concomitant underlying sinus node dysfunction. Older patients have different mechanisms at play that lead to hypotension and reduced cardiac output compared with younger ones.6,7 Reliance on rhythm monitoring to decide upon the utility of pacing in VVS may thus be misguided in some patients.24. However, given no clearly effective drug or drug combination,34,35 the association of episodes with bradycardia or asystole naturally makes one consider permanent pacing for selected individuals including those who are young (Figure 3). In this manuscript, we provide a critical review of the role of permanent cardiac pacing for VVS.
Pacing for Vasovagal Syncope
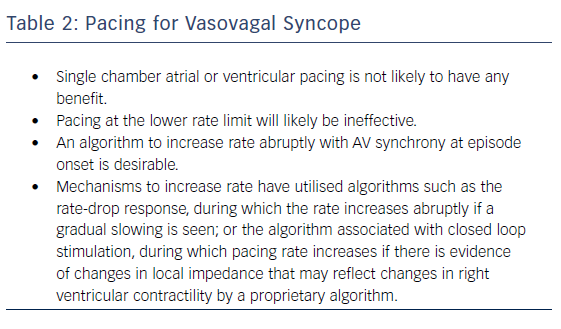
Even if bradycardia or asystole occur around the time of syncope, pacing may not prevent syncope if hypotension is profound and the cause for syncope (Table 2).36–38 However, pacing may be able to modulate the VVS episode if performed early in the onset of hypotension, perhaps at rates greater than the lower rate limit of the pacemaker. If pacing were to work, it would be unlikely to be effective if it were single chamber ventricular pacing or pacing at the lower rate limit. Because hypotension is likely to be a concomitant factor, AV sequential pacing at approximately 100 BPM would be desirable.39. However, it is uncertain what rate is best to pace and if this could offset the effect of vasodilatation enough to prevent loss of consciousness. Pacing too fast may also drop the blood pressure. Moreover, the type, timing and rate of pacing likely makes a difference; physiological (AV sequential) pacing would be more likely to provide haemodynamic support in a patient who has peripheral vasodilatation (Figure 4). The relationship of the timing of pacing to events that occur during the vasovagal reflex may determine success.24
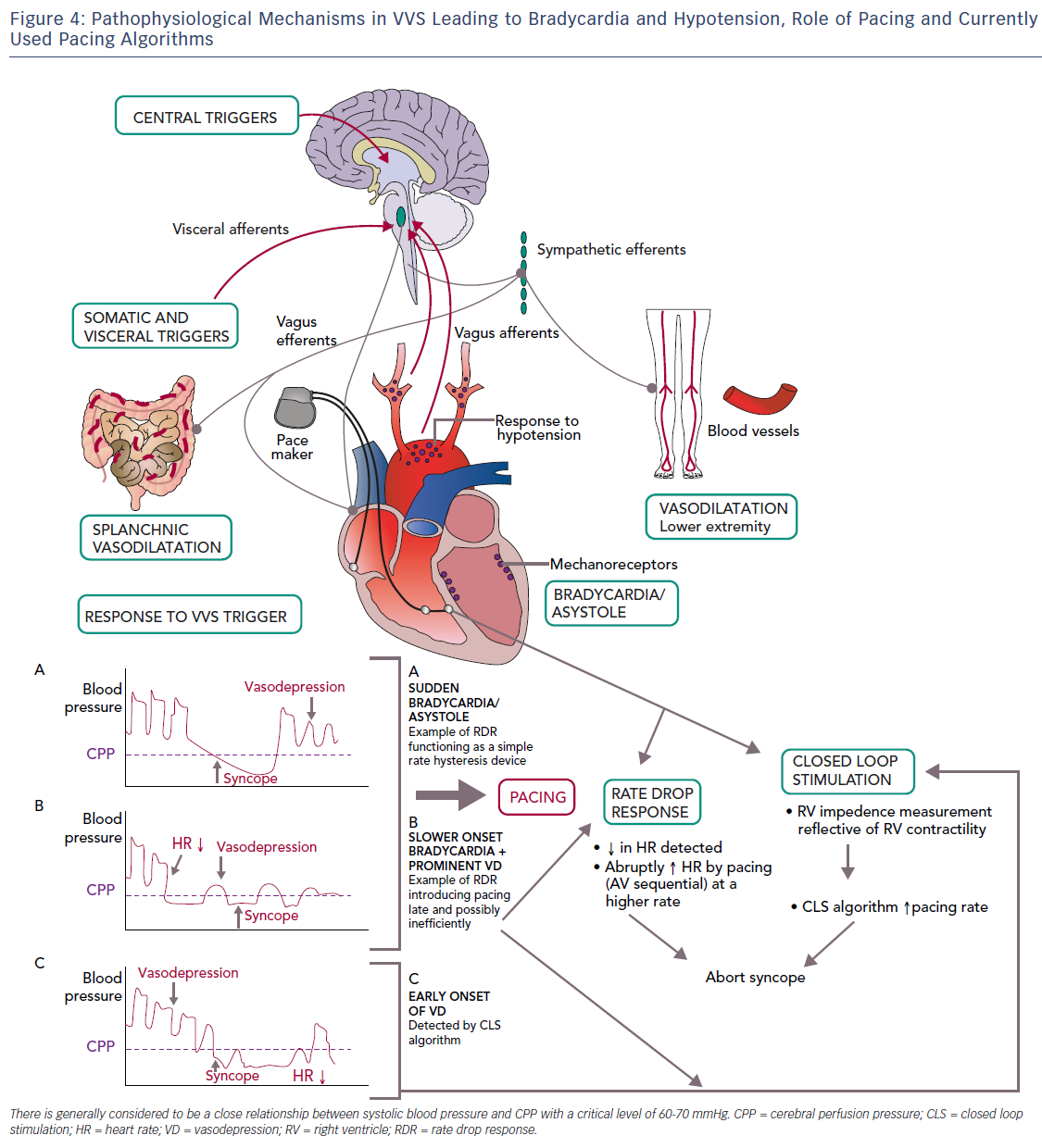
Is Pacing Necessary?
Although it may seem straightforward that pacing can help patients with syncope and bradycardia, cardiac pacing has a limited role in managing VVS. It is more than just the fact that data conflict regarding benefit of pacing to prevent syncope recurrence.40 Pacing may not effectively counteract vasodilatation, but may modulate hypotension if fast enough. It is important to know the frequency of recurrent VVS and also the need for future intervention. Those with VVS and documented asystole during tilt-table testing do not necessarily require any intervention.
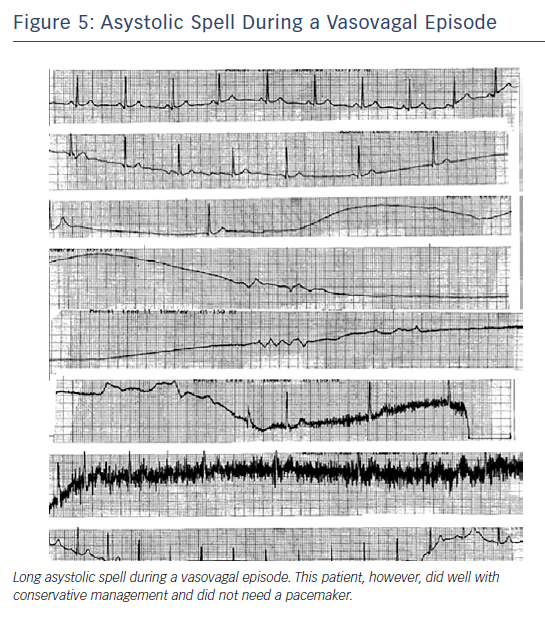
Carvalho et al. performed 2,263 consecutive tilt-table tests (utilising isosorbide dinitrate) in 2,247 patients with syncope finding that 149 had asystole (mean 10 seconds); 11 had asystole for ≥30 seconds with one episode lasting 63 seconds.41 Despite no pacemaker implant and with generally conservative management, after a median of 42 months of follow-up, four had syncope and only one had syncope-related injury, suggesting that pacing is not required for all individuals even if they have asystole on the tilt-table test41 (Figure 5).
Nevertheless, there are those who continue to collapse without warning and with asystole, for whom a pacemaker may prevent or reduce the frequency or severity of episodes (Figure 3).
Studies Evaluating Pacing
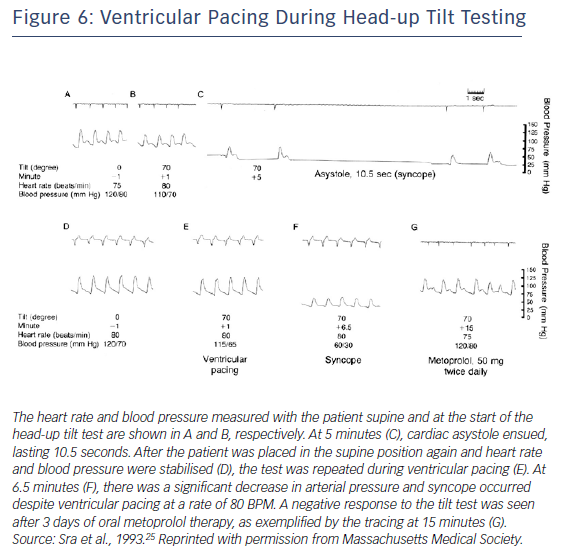
Early data supported use of pacing for VVS. Fitzpatrick et al. compared symptoms and haemodynamics in patients with VVS who had a positive tilt-table test response with evident bradycardia. Temporary AV sequential pacing during tilt-table testing with simulated rate hysteresis aborted five out of six syncopal episodes.42 Similarly, DDI pacing with rate hysteresis appeared effective in patients with cardioinhibitory VVS.43 However, Sra et al. evaluated patients who had bradycardia and/or asystole with hypotension during tilt table testing. For most patients, blood pressure declined much earlier (42 ± 29 seconds) than heart rate. With temporary AV sequential pacing, most patients continued to have a blood pressure drop with symptoms but to a lesser degree than without it (Figure 6).25 This finding likely has mechanistic implications with regard to patients employing counter-pressure manoeuvres or adopting a safer position such as sitting or lying down, thereby avoiding injury. It appears that rapid pacing faster than the lower rate may be necessary to provide adequate improvement in cardiac output and this reduce risk of syncope. Initial attempts at pacing were rather disappointing,44 but with a rate-drop response algorithm triggered by abrupt slowing in rate at the onset of VVS could lead to rapid AV sequential pacing for a selected time interval. Initial data looked promising.45,46
The North American Vasovagal Pacemaker Study (VPS I) randomised patients with three or more episodes of syncope and a positive tilt-table test (hypotension and relative bradycardia) to dual chamber pacing (lower rate of 60 BPM with rate-drop response) versus no pacemaker. Subsequent syncope occurred in 70 % with no pacemaker but only 22 % in the pacemaker group, a stunning 85 % reduction (CI [59.7–94.7 %]; p=0.00002). The mean time from randomisation to syncope was 54 days in the no-pacemaker group and 112 days in the pacemaker group.37 Since this study was not blinded, a placebo response to pacing could not be ruled out.
VASIS (Vasovagal Syncope International Study), a multicentre randomised study, compared DDI pacing with rate hysteresis to standard therapy in patients with three or more syncope episodes over 2 years and positive cardioinhibitory response at tilt-table testing. Only one patient (5 %) in the pacemaker group had syncope versus 61 % given standard therapy (p=0.0006).47
Similarly, Ammirati et al. performed a multicentre, randomised study of dual-chamber pacing with rate-drop response versus atenolol in patients ≥35 years who had three or more syncopal episodes in the preceding 2 years and positive tilt-table test showing relative bradycardia [Syncope Diagnosis and Treatment (SYDIT) study].48 There was a 4.3 % recurrence of syncope after a median of 390 days in the pacemaker group versus 25.5 % after median of 135 days in the atenolol group (OR 0.133; 95 % CI [0.028–0.632], p=0.004). Again, a placebo response to pacing could not be excluded.
To determine the impact of a placebo response, the VPS II (North American Vasovagal Pacemaker Study II) was performed. This multicentre, double-blinded trial compared VVS patients randomised to DDD pacing with rate-drop response or to pacemaker turned off (placebo). There was no significant reduction in syncope recurrence with active pacing indicating a potential contributory placebo effect of pacing but the study may have been underpowered to show a small difference as there was a 30 % reduction in syncope recurrence between groups (95 % CI [−33–63 %]; 1-sided p=0.14).36 Moreover, the follow-up was only 6 months and intense bradycardia documentation was not an entry criterion. These data were further supported by SYNPACE (The vasovagal Syncope and Pacing Trial), a multicentre randomised, double-blinded, placebo-controlled study of syncope positive tilt-table test patients who underwent pacemaker placement. There was no difference in syncope recurrence between those who had active DDD pacing with rate-drop response versus no effective pacing.49 SYNPACE also did not require a thorough effort to document intense bradycardia as an inclusion criteria. These issues prompted development of the ISSUE-2 registry.50
The ISSUE-3 (Third International Study on Syncope of Uncertain Etiology) trial, a double-blinded, randomised, placebo-controlled, multicentre study, included patients ≥40 years, with three or more syncopal episodes in the previous 2 years who had documentation of syncope with an implantable loop recorder (ILR) showing ≥3 seconds of asystole in a symptomatic episode or ≥6 seconds of asystole in an asymptomatic episode. Patients were randomly assigned to DDD pacing with rate-drop response or sensing only. With pacing, the risk of syncope recurrence was reduced 57 % (95 % CI [4–81]) from 57 % to 25 %; p=0.039).51
ISSUE-3, however, was not without issues. Patients who had asystole during tilt-table testing had less benefit from pacing. As the average age of enrollees was 63 years and only 44 % had a typical vasovagal/situational presentation, one must wonder if the benefit of pacing to prevent bradycardia was due to VVS or to sinus node dysfunction in this older population. While this point is worth considering, much thought and discussion went into distinguishing sinus node dysfunction from a vasovagal response as part of the publication; ultimately sinus node dysfunction was considered unlikely since there was no clinical difference between the patients who might have had sinus node dysfunction and those who did not. This issue was thoroughly argued before ISSUE-3’s publication. In another report of an older population with VVS, the Syncope Unit Project (SUP-2) pacing reduced syncope burden from 200 episodes per year to 11 episodes per year, a 95 % relative reduction in 2 years of follow-up.52
Impact of Closed Loop Stimulation
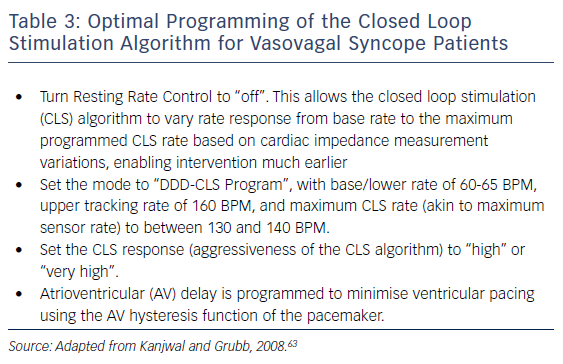
Until recently, rate-drop response was the most commonly studied algorithm for VVS,36,37,48,49,51 but pacing support, even at faster rates, may be too little and too late to counteract reflex vasodilation.37 Recent evidence points to the use of closed loop simulation (CLS) to initiate pacing at an earlier stage.24,53–59 CLS is a proprietary Biotronik algorithm, purported to measure intracardiac impedance during systole for each beat, but it actually measures local impedance in the right ventricle, which may relate to contractility. Influencing impedance is right ventricular volume. Based on this, the algorithm adjusts pacing rate dependent upon changes in measured impedance, such as may be noted early in the onset of a vasovagal event.55,60 How best to program the CLS algorithm to allow intervention early on in a vasovagal reflex, and thereby prevent the faint, is detailed in Table 3.
Reports using CLS to adjust pacing are not new and date from 2004. In an observational study, Kanjwal et al. followed patients with two or more syncopal episodes in the preceding 6 months, refractory to other treatments and evident asystole (>10 seconds) or severe bradycardia (<30 BPM) observed by implantable loop recorder or tilt-table testing. Compared to those who had pacing with rate hysteresis or rate-drop response, the CLS group had less recurrent syncope (83 % versus 59 %) and greater reduction in syncope burden (84 % versus 25 %, p=0.002).53
The INVASY (Inotropy Controlled Pacing in Vasovagal Syncope) study, a randomised study, compared DDD rate-adaptive CLS to DDI pacing in patients with recurrent VVS and a positive tilt-table test with cardioinhibitory response. Seven of nine patients randomised to DDI mode had recurrent syncope during the first year, whereas none randomised to DDD-CLS did.55 The long-term utility of CLS pacing was investigated in a prospective study that followed patients for 3 years comparing events before and after implant. During follow-up, 83 % of patients were asymptomatic; only five had syncope with CLS pacing.54
In a prospective randomised single-blinded multicentre study of patients with cardioinhibitory VVS (age 62 ± 14 years), DDD-CLS (versus DDD) pacing reduced syncope occurrence induced by tilt-table testing (30 % versus 77 %; p<0.001), reduced blood pressure drop during tilt-table testing and significantly delayed onset of syncope.57 In another report of patients (age 53 ± 5.1 years) with tilt-table induced cardioinhibitory response who were randomised to CLS “off” versus “on” for 18 months in a crossover design showed that CLS pacing was associated with fewer syncopal and presyncopal events (syncope: 2 versus 15; p=0.007; presyncope: 5 versus 30; p=0.004).58
The SPAIN (Closed Loop Stimulation for Neuromediated Syncope) trial, a recent, randomised, double-blinded, crossover study, enrolled patients ≥ 40 years old with high burden of syncope (five or more episodes or two or more episodes in the past year) and a cardioinhibitory response to tilt-table testing (bradycardia <40 BPM for 10 seconds or asystole >3 seconds). Patients were randomised to DDD-CLS versus sham DDI pacing (30 pulse/minute subthreshold) and crossed over at 12 months or when a maximum of three syncopal episodes occurred within 1 month. The proportion with ≥50 % reduction in syncopal episodes was 72 % (95 % CI [47–90 %]) with DDD-CLS versus 28 % (95 % CI [9.7–53.5 %]) with sham DDI mode (p=0.017). Overall, four patients in the CLS group passed out versus 21 in the DDI group. There was substantial improvement in time to first syncope in the CLS group (29 months versus 9 months; OR 11; p<0.0001). Following crossover, marked reductions in events were seen with DDD-CLS pacing in both groups. CLS resulted in a 37 % absolute risk reduction in time to first syncope (number needed to treat to prevent one syncopal episode was 2.7).59 Detecting changes in cardiac impedance measurements early using the CLS algorithm might provide prompt and aggressive heart rate support to prevent relative bradycardia or asystole and may modulate hypotension enough to prevent syncope. Thus, DDD-CLS pacing has been shown to be effective in a double-blinded trial.
To keep this in perspective, ISSUE-3, also double-blinded, showed statistically significant benefit for pacing versus sensing only modes. Additionally, the prospective, multicentre, observational Syncope Unit Project 2 (SUP-2) study validated a standardised guideline-based algorithm to select those for pacing.52,61 In this study, patients age >40 years with severe unpredictable recurrent reflex syncope having evidence for an asystolic response underwent dual-chamber pacing (often with a rate drop feature). Of 281 patients meeting inclusion, 137 received a pacemaker. At 3 years, the actuarial syncope recurrence rate was 20 % (95 % CI [12–30]), lower than those monitored by an implantable loop recorder (43 %, 95 % CI [29–57]; p=0.01). In our clinical experience, DDD pacing with rate-drop response, with or without additional medical therapy, can be effective as well.
The ongoing Benefit of Dual Chamber Pacing with Closed Loop Stimulation (CLS) in Tilt-induced Cardioinhibitory Reflex Syncope (BIOSync CLS) study (NCT02324920) a multicentre randomised, double-blinded, parallel trial, evaluating patients 40 years old and older with frequent VVS, will compare dual-chamber DDD-CLS pacing with placebo (pacemaker mode ODO). The study has a prespecified 2-year follow-up, with estimated completion in October 2019.62
Who Needs a Pacemaker?
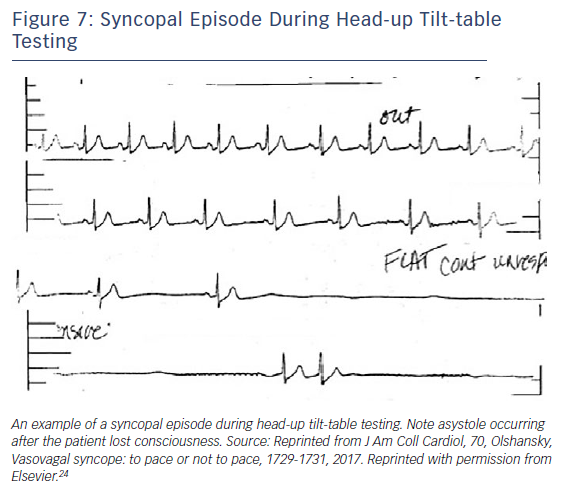
The fact that pacing can be effective in some patients with syncope does not mean that it is required for all patients. Moreover, even in patients with episodic asystole that is directly temporally related to the event itself and even if it is not associated with hypotension, pacing might still not be indicated (Figure 7). Since vasovagal episodes are common, pacing needs to be directed at the subset of patients who have recurrent episodes for whom pacing will abort the episode(s). This may include older (>40 years) individuals as well as those who experience frequent recurrences, debilitating consequence, repeated injury, limited prodrome and documented asystole.34 There is no specific reason a pacemaker would not be effective in an individual younger than 40 years, but careful consideration for an implant in a young patient is required.
Guidelines
Guideline and consensus recommendations agree in part, but are not fully aligned.34,40,63–66 The 2008 American College of Cardiology/American Heart Association (ACC/AHA) Pacemaker Guidelines gives a class IIb indication for pacing for symptomatic neurocardiogenic syncope associated with bradycardia documented spontaneously or by tilt-table test (level of evidence [LOE] B).63 Similarly, the 2017 ACC/AHA/Heart Rhythm Society (HRS) Guideline for the Evaluation and Management of Patients with Syncope recommends dual-chamber. pacing as reasonable for patients over the age of 40 years with recurrent vasovagal syncope and spontaneous pauses (class IIb, LOE B-R).33
The 2018 ESC Guidelines for the Diagnosis and Management of Syncope consider pacing is reasonable for patients over 40 years old with spontaneous documented symptomatic asystolic pauses >3 seconds or asymptomatic pauses >6 seconds due to sinus arrest, AV block or a combination (Class IIa, LOE B).26 These guidelines also recommend that pacing may be considered to reduce syncope recurrences in patients with tilt-induced asystolic response who are >40 years with recurrent frequent unpredictable syncope (Class IIb, LOE B), but advises against pacing in the absence of a cardioinhibitory response.
Similarly, the 2015 HRS Expert Consensus Statement gives a Class IIa (LOE B-R) indication for pacing in patients >40 years with recurrent, unpredictable syncope and a documented pause >3 seconds during clinical syncope or an asymptomatic pause >6 seconds.65 Pacing has a Class IIb, LOE B-R recommendation for paediatric patients with recurrent syncope with documented symptomatic asystole refractory to medical therapy.
The 2017 Systematic Review for ACC/AHA/HRS Guidelines concluded that the current evidence does not support pacing for patients with recurrent VVS and asystole.40 However, establishing a relationship between symptoms and severe bradycardia is essential before considering permanent pacing. This is easier said than done, as the role of hypotension in the reflex sequence complicates the picture. Prolonged ECG monitoring, usually by an ILR, may be useful,34 but still the mechanism of syncope could be due to hypotension.
Confounding Issues
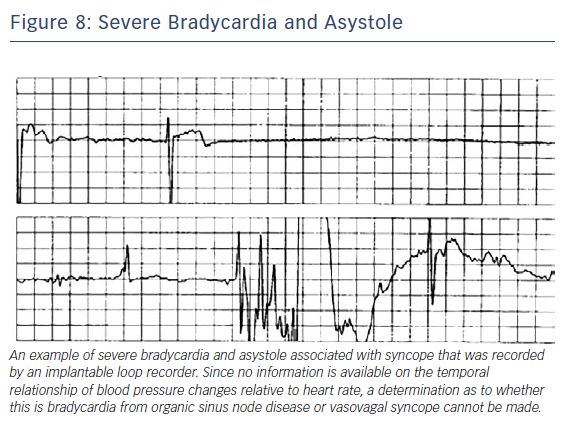
Older patients have a higher likelihood of sick sinus syndrome and consequent bradycardia rather than VVS and the two can be difficult to distinguish. Bradycardia recorded from a loop recorder could be problematic, as no information is available on temporal relationship of blood pressure changes relative to heart rate (Figure 8). Asystole on a tilt-table test, however, may not be a sensitive or specific indication of spontaneous asystole or the need for pacing but recurrent, frequent and severe VVS episodes could justify implanting a pacemaker.
Further complicating matters, a small subgroup of patients may have idiopathic AV block during syncope detected by ECG monitoring. These patients usually have a structurally normal heart, a normal ECG, no sign of conduction system disease on ECG or electrophysiology study, a normal tilt-table test and low levels of adenosine. This form of syncope generally occurs with no prodrome and is mediated by adenosine. These patients will likely need cardiac pacing.67,68
Young patients with VVS and asystole may have a specific trigger, may have rare episodes of syncope and may not benefit from pacing therapy. Thus, caution is advised with use of long-term pacing in individuals younger than 40 years, especially since living with a pacemaker at a young age can be difficult and can lead to long-term complications. However, younger patients with frequent, debilitating, recurrent asystolic vasovagal syncope unresponsive to any other therapy or unable to be treated in any other way may indeed be candidates for pacing.
Radiofrequency ablation of ganglionic plexi in the right atrium, near the superior vena cava and sinus node, inferior vena cava, near the coronary sinus and AV node, and in the left atrium near the floor and near all four pulmonary veins with the aim of abolishing vagal efferent activation during VVS (cardioneuroablation) has shown promise in early observational studies,69-71 but larger, controlled studies with long-term follow-up are needed to confirm the safety and efficacy of this procedure.
Knowledge Gaps
Despite the trials outlined above, several knowledge gaps remain:
- What is the mechanism responsible for VVS and how can it be best counteracted?
- Is there a way to abort an episode of VVS before it goes to completion?
- Is pacing useful for those under the age of 40 years with recurrent VVS associated with severe bradycardia and/or asystole?
- Is there a role for concomitant medical therapy with pacing for VVS patients?
- Why does the reflex reset itself after a few seconds, and how?
- Which patients with VVS over 40 years of age require and benefit from pacing?
- Does tilt-table testing combined with ILR monitoring provide better insights into identifying the best candidates for pacing in VVS?
- Is tilt-table testing required to evaluate the need for pacing in VVS?
- Can pacing algorithms other than CLS benefit select patient subsets?
- How is it best to programme the pacemaker?
Conclusion
VVS is a common problem due to a ubiquitous, counterintuitive reflex. Initiating factors may affect sympathetic activation. While most patients can be managed conservatively without the need for specific medical interventions, emerging evidence indicates that pacing may reduce recurrent syncope for select patients, especially if episodes are frequent, recurrent and otherwise difficult to manage.
The pacing algorithm, rate, type and timing of pacing, with respect to onset of the reflex, may be critical to prevent fainting. Pacing should be considered especially if syncope occurs concomitant with a cardioinhibitory response. Tilt-table testing may help quantify the heart rate and blood pressure responses temporally associated with vasovagal syncope. By detecting local impedance in the right ventricle which may relate to contractility, CLS may assess autonomic function and improve the timing for onset of pacing. DDD pacing with rate response gauged by the CLS algorithm appears to be the current best alternative to detect the need for pacing and prevent recurrent episodes in VVS. However, compelling data also support the use of pacing with rate-drop response for patients selected by the presence of asystolic episodes.