Ventricular tachycardia (VT) is a life-threatening sequela found in patients with cardiomyopathy. ICDs can terminate ventricular arrhythmias, but recurrent device shocks lead to reduced quality of life and are associated with higher mortality.1–5 Radiofrequency catheter ablation has emerged as an effective treatment for VT refractory to anti-arrhythmic therapy and has also been shown to reduce ICD shocks.6,7 However, in the presence of structural heart disease, the long-term recurrence rate of ventricular arrhythmias has been shown to be greater than 50% across multiple trials, with complication rates ranging from 6 to 10%.7–15 In addition, the cost to the healthcare system of initial ablation and hospitalisation per patient has been estimated at C$20,642 (£11,920); 995% CI C$11,773–44,741 [£6,798–£25,836]). Taken together, these numbers signal a need for treatments that are more efficacious and cost-effective.16
Treatment failure is likely the result of multiple potential factors. Endocardial radiofrequency ablation has limited ability to penetrate deeper arrhythmogenic substrates in the ventricular myocardium, particularly when disease processes alter the myocardial composition with replacement fibrosis, fat, calcification, or overlying chronic thrombus.
Even when percutaneous epicardial access can be safely obtained in patients without prior cardiac surgery, ablation energy delivery is often limited by epicardial fat and risk of coronary and phrenic nerve injury.9,17–19 While various alternative techniques have been devised to allow greater access, including transcoronary ethanol ablation, needle catheter ablation and bipolar ablation, these methods still rely on invasive techniques to achieve a permanent and sufficiently transmural lesion that encompasses critical VT circuitry.17,20–22 The clinical VT may also be too unstable to adequately map and delineate during catheter ablation, and there may be multiple VT circuits and substrates that preclude adequate mapping and treatment. These factors point to the complex architecture that often underlies VT substrate.
Stereotactic body radiotherapy (SBRT) is a non-invasive ablation modality, originally developed for the focal treatment of solid malignancies. It is now being applied to cardiac arrhythmias with promising early results. This widely used technology uses high-energy photons generated from many radiation beams directed at different angles to concentrate ablative energy in any pre-defined zone within the body.11
SBRT should overcome many of the limitations of catheter ablation, as it can target substrates that are too extensive or inaccessible using catheter ablation. However, the freedom to non-invasively designate and deliver treatment volumetrically and the use of SBRT as the ablation energy source brings new challenges and considerations.
Historical Background to Stereotactic Radioablation
Stereotactic radiotherapy was first conceived by the neurosurgeon Lars Leksell in the 1950s as a non-invasive method for treating inaccessible lesions deep in the brain, particularly arteriovenous malformations (AVMs).23 Termed stereotactic radiosurgery (SRS), the technology was rapidly adopted for the treatment of brain tumours and was used as a non-invasive outpatient procedure with short recovery times.24 While initially restricted to the brain by limited technological capability to accurately target and account for physiologic motion, advancements in imaging, treatment planning and radiation delivery systems have allowed the same technology to be adapted for treatment of tumours throughout the body in a technique called stereotactic body radiation therapy (SBRT).25 SBRT has also been used clinically to treat numerous benign conditions, including keloids, heterotopic ossification and trigeminal neuralgia.26,27 Non-malignant diseases that may benefit from the therapy are being investigated, including hypertension treated via renal arterial denervation.28
Mechanism of Tissue Injury and Pre-clinical Validation
During SBRT, high-dose radiation is delivered via numerous non-coplanar beams that converge on a single target with sub-millimetre accuracy.29,30 In contrast, conventional radiotherapy uses no more than several beams at one time and the radiation is fractionated into small dosages delivered over weeks to months to avoid collateral injury to adjacent organs. Because SBRT distributes radiation across many beams at different angles, the dose delivered to any one region of healthy tissue is minimised, which allows for treatment to be given in either one or several fractions.
The primary mechanism of radiation-induced cell death is from ionisation and free radical production, which leads to the accumulation of double-strand breaks in DNA that trigger cell cycle arrest and cell death. There is growing evidence that SBRT also works through additional indirect mechanisms owing to the higher dose, mainly through damage to tissue vasculature, leading to cell hypoxia and necrosis.24,31 To this end, radiation-induced vascular changes in tumours have been observed in the hours or days after treatment, and pre-clinical studies have shown additional cell death aside from the direct effects of radiation.31,32 These mechanisms have not been fully elucidated and there remains controversy over their exact role in SBRT.
Most of the literature has focused on tumour biology, and less is known about mechanisms of injury in normal tissue and especially arrhythmogenic cardiac tissue. Since radiation in cardiac SBRT is directed at non-dividing myocytes, the mechanism of action is likely to be different than that for tumours. However, there have been studies that have investigated single-fraction whole heart irradiation in animal models, and these demonstrated dose-dependent myocardial degeneration and fibrosis progressing from epicardial tissue to full transmurality in the months after irradiation at doses of 20 Gy and higher.33–35 Evidence of a reduction in capillary density was shown to precede that of myocardial degeneration, suggesting the early and prominent role of vascular injury in radiation-induced damage.34
Though not entirely translatable given differences in treatment delivery, these findings are similar to those observed in the proof-of-principle animal studies that have demonstrated successful AV nodal and pulmonary vein ablation following SBRT.36–39 In these studies, treatment effect was associated with myocyte necrosis and microvascular injury and, in particular, target histology (obtained after 3–6 months) consistently demonstrated radiation-induced fibrosis at the site of treatment with minimal effects outside of the target volume and no evidence of injury to surrounding tissues, including the trachea, oesophagus, lungs and phrenic nerves. Tissue sections were notable for severe myocyte architectural disruption and necrosis, along with severe vasculitis in intramyocardial vessels. However, these assessments of complications were still within a relatively short post-treatment timeframe, as radiation effects may not manifest for years.
Dose-finding across these studies supported a threshold of 25 Gy delivered as a single fraction, as effective at creating myocardial fibrosis and associated conduction block, but dosages of up to 35 to 40 Gy had no complications associated with radiation.36,39,40 The timeline of measured electrophysiologic effect, specifically conduction block, varied but was typically months, though shorter durations were observed for higher dosages of 35 to 40 Gy. In contrast, as will be discussed, clinical electrophysiologic effects appear to occur significantly sooner. Based on these results and experience gleaned from other applications of SBRT in oncology, clinical studies have used 25 Gy for treatment. Nonetheless, the optimal dose regimen has yet to be elucidated. Additionally, the pre-clinical studies described were done on normal cardiac tissue; therefore, the effect of SBRT on myocardial scar biology and electrophysiology remains poorly understood.
Clinical Application to Treatment of Ventricular Tachycardia
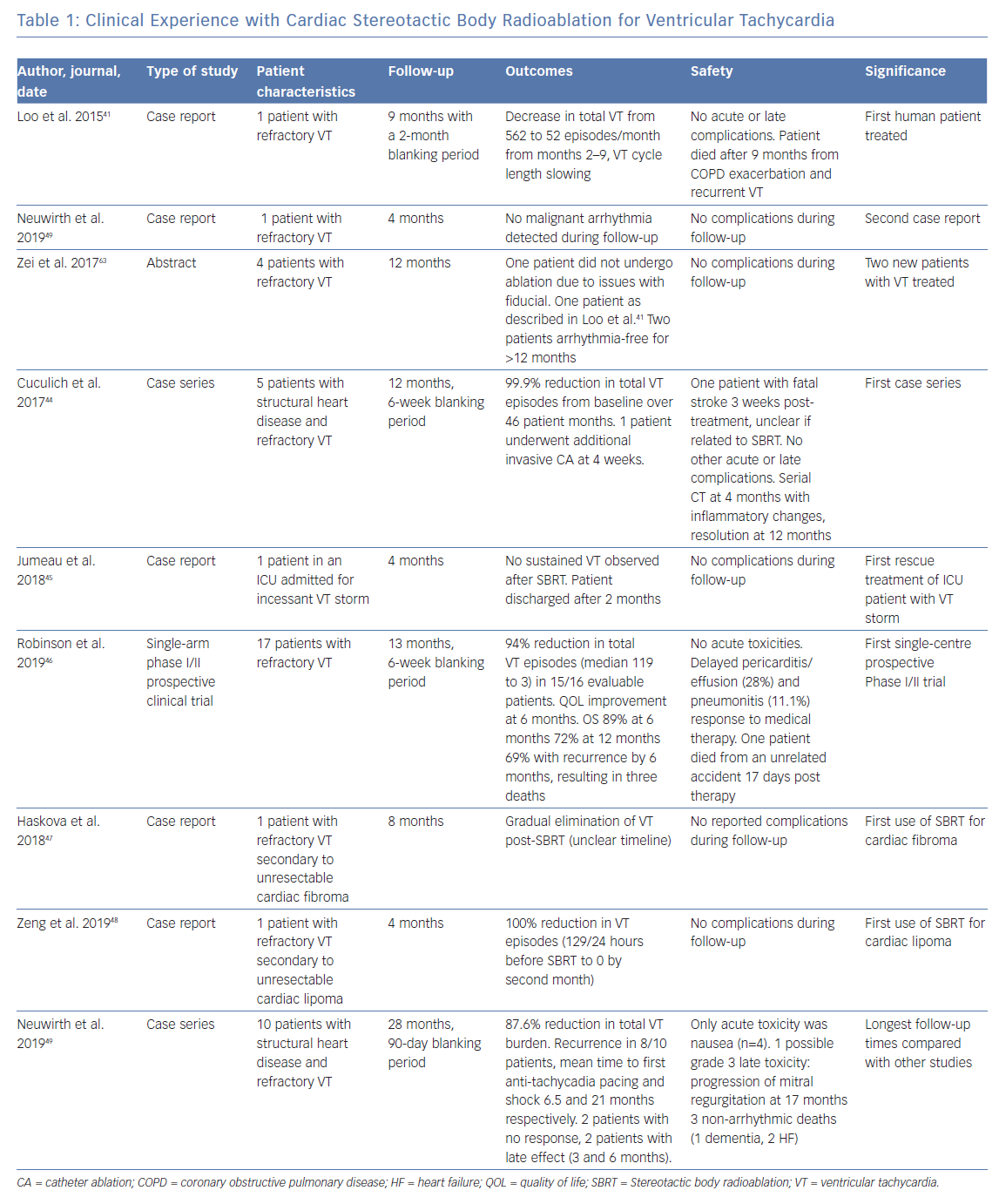
Clinical experience with cardiac SBRT as a treatment for ventricular tachycardia has focused on patients who have failed anti-arrhythmic and conventional catheter ablation therapy. To date, more than 50 patients have been treated worldwide, with published data including a total of 43 patients.41–49
The first reported use of SBRT for treatment of VT was published by Loo et al. in 2014. It demonstrated a transient decrease in VT episodes for 7 months after a 2-month blanking period after irradiation with 25 Gy.41 No acute or late complications were observed, but at 9 months, recurrent VT occurred in the context of and exacerbation of chronic obstructive pulmonary disease (COPD), ending in death. This seminal study was followed by several more case reports detailing the efficacy of cardiac SBRT, with details summarised in Table 1.42,43,45
The next representative study was a case series conducted by Cuculich et al. involving five patients treated for refractory VT, with a resulting 99.9% reduction in VT episodes from baseline, again without complications through a total follow-up period of 46 person-months.44 The largest study to date, a Phase I/II clinical trial that enrolled 19 patients, was published by Robinson et al. in 2019, and demonstrated a reduction from baseline in median VT episodes for 15 out of 16 patients followed for a median time of 13 months, with associated improvement in quality-of-life measures.46
Most recently, Neuwirth et al. published a case series of 10 patients with structural heart disease and refractory VT, and observed a 87.6% reduction in total VT burden after a 90-day blanking period.49 In this cohort, two patients showed no response to SBRT, two had late response at 3 and 6 months, and eight of the 10 patients had recurrence with mean time to anti-tachycardia pacing and shock at 6.5 and 21 months respectively.
Collectively, these studies appear to show a dramatic reduction in device-detected VT burden following therapy after a set blanking period. In general, side-effects were mostly mild, with the most significant events involving one report of pericarditis which was managed conservatively with anti-inflammatories, one case of valvular disease progression, two patients with self-resolving pneumonitis, and five with delayed pericardial effusions.46,49 No deaths resulted from the treatment itself, though many patients later experienced recurrence, with some resulting in death.46 Histology was obtained for one patient who suffered from stroke unrelated to the treatment during follow-up, which demonstrated prominent ectatic blood vessels at the interface of dense scar and viable myocardium, as has been described in pre-clinical studies, though without evidence of acute vasculitis or tissue oedema.44 There was no evidence of acute myocyte necrosis, haemorrhage, or acute inflammation. The results of the published data are summarised in Table 1.
SBRT has been associated with a remarkable reduction of VT burden. However, a small proportion of patients failed to demonstrate this. Further study to understand the mechanisms behind a lack of response will be critical. Furthermore, it is plausible that further understanding of the mechanism of action and optimisation of the treatment protocol will extend the duration of treatment effect.46 It should also be emphasised that these findings were limited to patients who had already failed conventional therapy, and it is not known how effective cardiac SBRT is for patients with less refractory disease.
In addition, the timescale of initial treatment effect and the parameters that modulate it are not well understood. Investigators have generally incorporated a multi-week blanking period post-SBRT to allow for late radiation fibrosis to take effect in accordance with the suggested mechanism of action. However, several notable discrepancies in the clinical experience point towards additional mechanisms at play. For example, Jumeau et al. used cardiac SBRT to induce successful resolution of VT storm in the acute intensive care unit setting, with no recurrence of sustained VT after treatment, as did Neuwirth et al. in select patients.45,49 Robinson et al. also noted treatment efficacy in most patients within 6 weeks, prior to the several-month time window observed in the aforementioned pre-clinical studies.46
Conversely, Neuwirth et al. reported a lack of response in two patients and delayed treatment effect in two more patients, which they attributed to smaller target volume and discontinuation of anti-arrhythmics before SBRT.49 Additional studies providing more clinical data may help delineate the best treatment parameters as well as the optimal medical management strategy peri-ablation. Further research is also needed to characterise the progression of fibrosis in relation to treatment effect, as well as contributions from vascular injury, which has been shown to occur more acutely following radiation in the pre-clinical setting.
In terms of safety, acute and subacute toxicities appear uncommon and have been limited to several cases of pericarditis, pneumonitis and delayed pericardial effusions which were managed conservatively. However, the long-term side-effects of treatment are still under investigation. Another potential concern, though not yet seen clinically with cardiac SBRT, is cardiac device malfunction after radiotherapy, with estimates that range from 3–7% of cases and correlating with radiation beam energy.50,51 Given that cardiac SBRT involves high-dose ablative radiotherapy, device malfunction is a concern; nonetheless, the disturbances that have been described typically manifest as transient device interferences occurring only during irradiation or resets to back up settings. Given the need for further delineation of the risk profile of cardiac SBRT, we recommend careful peri-SBRT monitoring of device function.
SBRT Treatment Planning and Delivery
Successful implementation of SBRT hinges on numerous factors, including correct identification of the intended target, design of a radiotherapy plan that prioritises dosing to the target while sparing adjacent critical organs, and accurate radiation delivery, which requires collaboration from a multidisciplinary team of radiation oncologists, cardiac electrophysiologists, physicists, dosimetrists and therapists. Pre-treatment imaging, including CT or MRI, is required to delineate the arrhythmogenic substrate, after which an individualised treatment plan is generated using target contours drawn by a physician followed by computerised dosimetry planning.17–18 During planning and treatment delivery, compensation for cardiorespiratory motion is a unique consideration for cardiac applications of SBRT.
Patient Selection
While early results have been promising, cardiac SBRT remains under investigation and is indicated in patients who have intractable arrhythmia refractory to drug escalation and catheter ablation. As a non-invasive outpatient procedure that is not limited by substrate geography, it is particularly suited to patients with significant comorbidities who are unlikely to tolerate prolonged general anaesthesia or hospitalisation or have inaccessible arrhythmogenic substrate.
Determining the Arrhythmogenic Substrate
Cardiac SBRT depends on characterisation of both the anatomic and electrophysiologic topology of the arrhythmogenic substrate to inform the creation of an accurate 3D target volume. Cardiac imaging with CT, MRI and ECG provide an assessment of the structural correlate for VT circuits while 12-lead ECG, electroanatomic mapping (EAM) and multi-electrode electrocardiographic imaging (ECGI) provide valuable information on VT exit sites and isthmuses.
Most patients who have received cardiac SBRT have undergone prior ablation and previous EAM studies provide important information for SBRT treatment planning. If EAM was able to define an ablation target, but technical reasons precluded effective delivery of ablative energy, the mapping information may be quite helpful. ECGI or 12-lead ECG during non-invasive programmed stimulation can be useful where available, comprising a surface-based multi-electrode system that can record and reconstruct the heart’s electrical activity onto a CT-generated 3D anatomic model. In conjunction with non-invasive programmed stimulation through patient implanted devices, it has been used to map activation in VT.44 Currently, ECGI provides data primarily pertaining to epicardial electrical activity; in cases of endocardial or intramural substrate, substrate location may still be accurately inferred from epicardial mapping data, but this remains an area that is being researched.25
In patients with structural heart disease, options for imaging anatomic scar include MRI, cardiac CT, and radionuclide imaging. Cardiac MRI is the current gold standard for assessing ventricular scar (as identified by late gadolinium enhancement), but usage may be limited in patients with non-compatible ICDs and in situations where image quality is perturbed by device artifacts.52,53 In these situations, cardiac CT can be a valuable alternative, as it is not only effective at characterising detailed cardiac anatomy due to its high spatial resolution, but can also define putative arrhythmogenic substrates, which often localise to sites of significant wall thinning, fibrosis, fat, and calcium.54–6 Radionuclide imaging can also be obtained using single photon emission CT (SPECT) or PET, both of which are commonly used to detect silent ischaemia, although these modalities have poor spatial resolution and often require integration with other imaging modalities.
Creation of the Treatment Volume
After target delineation, the next step in planning involves creating the target volume and determining the patient set-up required for treatment delivery. The patient is brought to a radiation oncology suite to undergo simulation, during which they are immobilised in the position they will be in when they receive radiation, and imaging is performed to simulate their anatomy during treatment. Several devices may be used to fix the patient in a reproducible position, including a vacuum-assisted cushion shaped to the patient’s body. Once the patient is positioned appropriately, a free-breathing planning CT is obtained, which serves as the anatomic reference upon which a 3D target is contoured. Where available, a respiratory-gated 4D CT may also be obtained, which comprises a series of reconstructed CT scans corresponding to different phases of breathing and provides information about target excursion throughout the respiratory cycle. A composite of these scans is fused with the free-breathing planning CT to create an adjusted planning image set.
Following the simulation, the electrical and anatomical information must be registered with the planning CT. The radiation oncologist, in consultation with the electrophysiologist, uses anatomic scar characterisation using MRI, CT, SPECT, and/or echocardiogram together with electrophysiologic data derived from EAM, ECGI, and/or 12-lead ECG as a guide for contouring the target on the planning CT. This is done on a separate software platform. The contouring process can be time-consuming owing to factors such as the manual comparison of imaging studies and mapping data, and consideration of adjacent radiosensitive organs, device lead insertion sites, valvular structures, conduction systems and the phrenic nerve.
Cardiorespiratory Motion Compensation
It is important to ensure that the treatment volume encompasses the target during cardiorespiratory motion. Since the heart contracts with a ‘wringing’ action with limited positional displacement, most of the heart’s translational movement can be attributed to the respiratory cycle, particularly at sites where myocardial contractility is reduced due to scarring.17
Specific cardiorespiratory compensation methods depend on the treatment device used and fall broadly into two categories. One strategy is to minimise motion as much as possible (using immobilisation and compression devices) and to contour a larger target volume to encompass the entire target location as seen on a respiratory-gated 4D CT and where available, a cardiac-gated CT.57
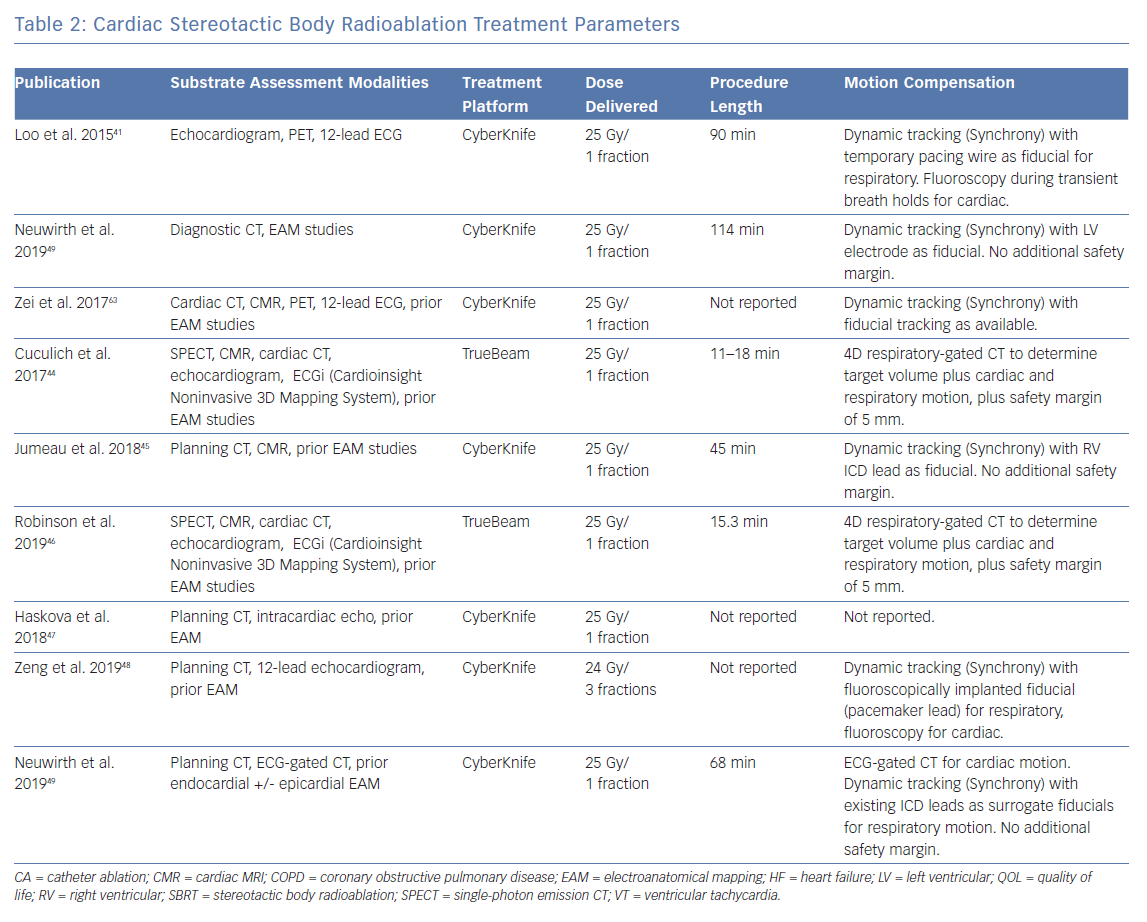
Another approach is to use a system capable of tracking motion and delivering radiation based on gating. In this method, a fiducial marker – which can be an existing device component or an implanted gold seed – is used to orient the radiation beam, serving as a trigger to switch the beam on and off as it moves in and out of a preset location. Another way is to use a linear accelerator mounted on a roving robotic arm that can move freely and synchronise with the target in real-time using an internal respiratory tracking system. The roving robotic arm allows the linear accelerator to access more oblique angles and the respiratory tracking system uses continual imaging of fiducials to align the radiation beam with the motion of the target.58 This latter approach is provided by the CyberKnife (Accuray) delivery platform. A summary of the motion compensation approaches that have been used is shown in Table 2.
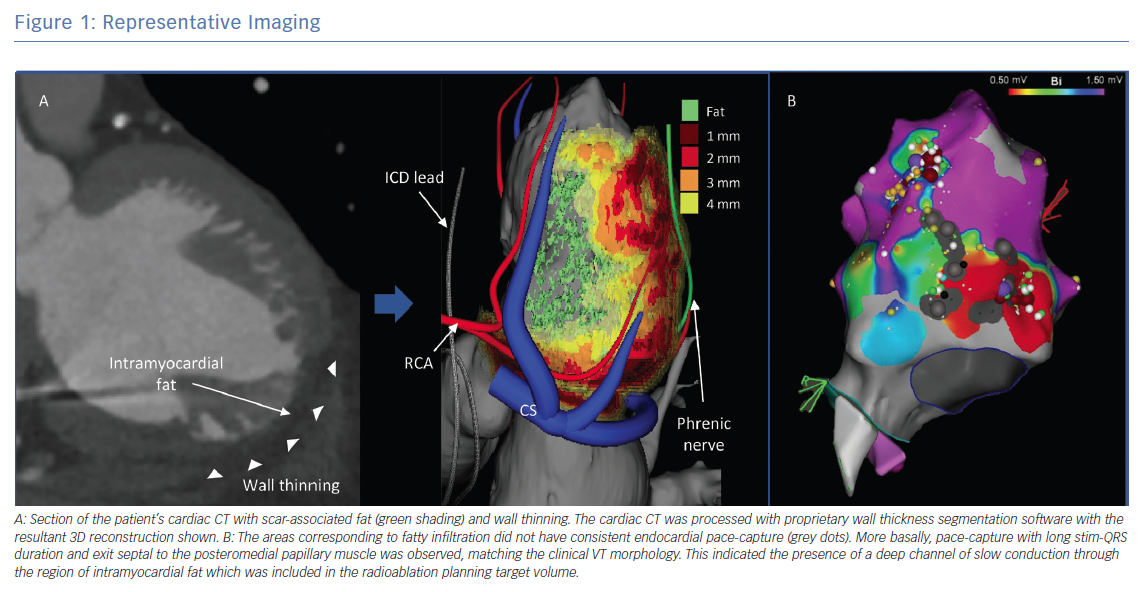
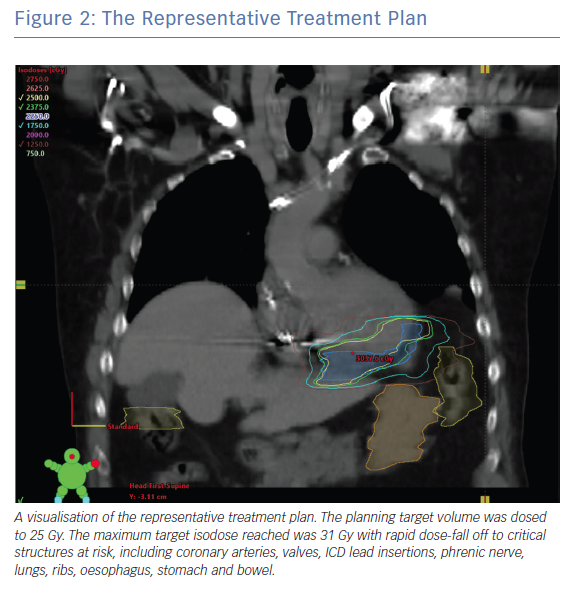
Figure 1 shows a representative example of the multimodality treatment planning required for cardiac SBRT. In this example, the patient presented with recurrent VT in the setting of ischaemic cardiomyopathy that was unresponsive to quinidine and amiodarone as well as three attempts at endocardial catheter ablation. Cardiac-gated CT was obtained to define detailed cardiac anatomy, particularly areas of wall thinning and myocardial scar components, as well as critical collateral structures (Figure 1A). Wall thickness segmentation was subsequently performed on the cardiac-gated CT, with image processing done using MUSIC software (Liryc-Université de Bordeaux/Inria Sophia Antipolis), which has been shown to accurately colocalise regions of wall thinning with voltage-defined scar.59 Also shown is the 3D reconstruction that was created using this method. The patient’s previous EAM is shown for side-by-side comparison. It demonstrates areas of dense and patchy scar in the basal to mid inferoseptal left ventricle, corresponding to fatty infiltration (Figure 1B). The radiation treatment plan and target volume were constructed by manually superimposing this information onto a treatment planning CT (fused to a respiratory-gated 4D-CT; Figure 2). The radiation was calculated to a target isodose of 25 Gy with a rapid dose fall-off.
Treatment Delivery
On the day of treatment, the patient only needs to spend a few hours at the centre. After check-in, the patient is assessed by the radiation oncologist and electrophysiologist and is then positioned according to the parameters determined at the time of the simulation. Treatment typically takes 10–15 minutes, after which the patient undergoes a period of post-treatment monitoring before they are discharged.
Depending on the institution, a variety of radiation delivery platforms are available. The treatment systems that have been used thus far include the CyberKnife and the Varian TrueBeam/Edge (Varian) (Table 2). Some platforms use dynamic target tracking, although it is unclear how much this improves targeting accuracy.42,45,49 Undoubtedly, treatment times with these platforms are significantly longer.60 In comparison, systems for which continuous fiducial imaging has not been applied, such as the Varian TrueBeam/Edge, are comparably more time efficient and instead use X-rays taken at regular intervals throughout treatment to ensure correct patient alignment.60 In fact, the increased treatment time necessitated by a motion tracking approach may introduce treatment inaccuracies and/or reduced treatment efficacy. Further investigation is needed for this.
Follow-up
Follow-up consists of regular surveillance by the electrophysiologist and radiation oncologist. These include ICD checks and post-treatment imaging with transthoracic ECG and cardiac CT to monitor efficacy and safety.
Our Approach
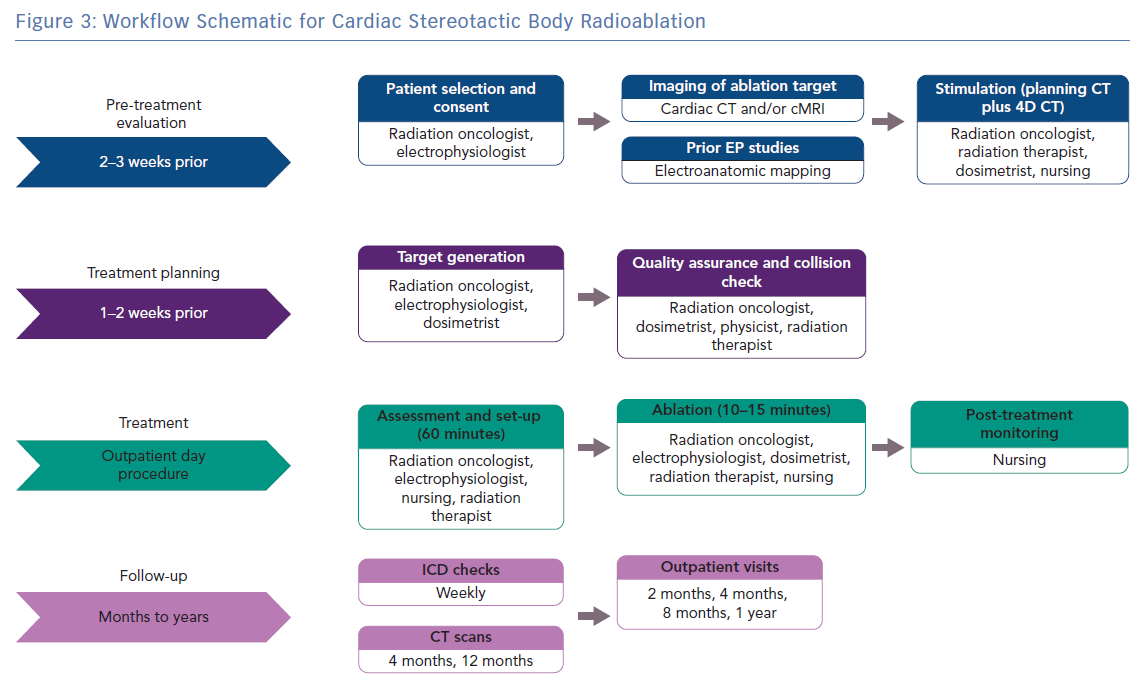
Our approach to treatment planning, delivery, and follow-up is summarised in Figure 3. In general, target definition is achieved using a combination of cardiac-gated CT and EAM. Cardiac and respiratory motion is visualised on both cardiac-gated CT and respiratory-gated 4D CT. Motion compensation is done by targeting the substrate throughout the combined motion envelope during treatment planning, and then by checking consistent alignment of anatomy with fiducials via serial imaging (on cone-beam CT and periodic triggered kV images) on the day of treatment. Target definition is done jointly by radiation oncology and electrophysiology and the full treatment planning takes 1–2 weeks. Patients are monitored after treatment for any complications and then followed with sequential ICD checks, imaging and clinic appointments.
Cost-effectiveness
There are an estimated 4 million–5 million cases of sudden cardiac death per year worldwide, of which a substantial proportion result from ventricular arrhythmias.60 Little data exists regarding the cost-effectiveness of available VT treatments, but the charges associated with initial catheter ablation and the subsequent hospital stay have been estimated at C$20,642 (£11,920).16 The relationship between charges, which reflect the cost to the healthcare system and true cost has not been explored, but it would not be unreasonable to assume that the two are closely associated, particularly in Canada’s publicly funded healthcare system. Regardless, because cardiac SBRT is an outpatient treatment that obviates the need for anaesthesia or hospitalisation, its adoption may lead to significant cost savings compared with catheter ablation.
Although there is no data on cardiac SBRT, the charges associated with lung SBRT have been estimated at $10,616 and $8,042 (£8,150 and £ 6,174) over 3–5 fractions in US and Canadian studies respectively; those for cardiac SBRT may be lower given that it is administered in one fraction.61,62 Additionally, SBRT is widely available worldwide, with recent data confirming a total of 11,568 radioablation devices installed and in active use, which may arguably present a lower barrier for patient access than treatment in electrophysiology labs capable of performing complex VT ablation.17 Nevertheless, a direct comparison of charges as well as true cost between cardiac SBRT and catheter ablation is not yet available and the patient population for which this novel procedure is indicated is limited to those who have not responded to conventional therapy. As we gain more clinical experience and data from longer clinical follow-up that can help assess efficacy and safety, such a comparison of cost-effectiveness may become possible.
Future Directions
Although cardiac SBRT is a promising novel treatment modality for medically refractory VT, significant clinical and technical questions need to be addressed. For the former, these include elucidating the underlying mechanism of radiation-associated treatment effect and its long-term durability. For the latter, further work needs to be directed towards identifying the best protocol for treatment planning, including substrate characterisation, multimodality integration, motion compensation, as well as treatment delivery. The ideal standardised approach should improve efficiency and efficacy, with an overall goal of extending the duration of treatment effect.
Future studies would benefit from more detailed follow-up to examine the time course for anti-arrhythmic effect in the blanking period. Similarly, an improved understanding of the pathophysiology of radiation-induced conduction block and tissue injury in normal myocardium and myocardial scar is greatly needed. Standard ICD programming should also be used (particularly if an endpoint of device-detected VT burden or ICD therapies is used). A registry to enable follow-up beyond enrolment in a clinical trial and to standardise approaches for treatment delivery would be helpful in this emerging therapy. An additional benefit that a registry would provide is tracking of real-world safety and efficacy data. Ongoing collaboration among radiation oncologists, electrophysiologists, cardiac imaging specialists, as well as basic science researchers will help drive this progress.
Conclusion
While early results have been promising, cardiac SBRT remains an investigational protocol that is indicated in patients who have intractable arrhythmia that does not respond to drug escalation and catheter ablation. As a non-invasive outpatient procedure that is not limited by substrate geography, it is particularly well-suited to patients who would not tolerate anaesthesia or prolonged hospitalisation and have poorly accessible substrate that is still well-defined. Clinical experience has demonstrated good short-term efficacy with minimal adverse effects; however, additional studies are needed to investigate its long-term efficacy and side-effects, its mechanism of effect and its cost-effectiveness.
Clinical Perspective
- The use of stereotactic body radioablation for treatment of medically refractory ventricular tachycardia provides distinct advantages which include non-invasive ablation, outpatient treatment and the ability to target larger substrates and sites otherwise inaccessible by radiofrequency catheter ablation.
- The mechanism of action is not well understood, but may be due to a combination of subacute vascular damage and late radiation fibrosis with a timeline of treatment effect that typically ranges from weeks to months but has been observed in the acute setting.
- The clinical experience includes more than 50 treated patients and collectively demonstrates dramatic short-term reduction in device-detected VT burden with minimal acute to subacute side-effects but unclear long-term safety and efficacy.
- Successful treatment planning requires close multidisciplinary collaboration to integrate anatomic and electrophysiologic target delineation, creation of the target volume and treatment plan, delivery of ionising radiation and follow up in the outpatient setting.
- Future research should aim to further elucidate the pathophysiology and timeline of radiation-induced anti-arrhythmic effect, define the ideal parameters of treatment, provide data on long-term safety and efficacy, and determine the cost-effectiveness of this novel treatment modality.