Atrial fibrillation (AF) is the most common cardiac arrhythmia and the prevalence is increasing every year; it is expected to affect 12 million Americans by the year 2030.1 A significant portion of patients fail to maintain sinus rhythm with use of anti-arrhythmic drug therapy and are referred for catheter ablation. Ablation is currently indicated for patients with paroxysmal (class I) or persistent (class IIa) AF who are intolerant or refractory to drug therapy.2 Cryoballoon (CB) ablation has emerged as an effective and alternative treatment option to traditional point-by-point radiofrequency (RF) ablation.3,4 In addition to the traditional major complications associated with AF ablation – stroke, cardiac tamponade and atrial oesophageal fistula (AEF) – CB ablation is also associated with phrenic nerve and bronchial injury. Knowledge of the close anatomical relationships between the pulmonary veins, the right phrenic nerve, oesophagus and the bronchial tree is critical to understanding why these structures are prone to collateral injury during CB ablation. Additionally, techniques to minimise the cryoablation dose, such as titration of freezing time utilising a time-to-isolation (TTI) strategy, can also help minimise collateral damage and, therefore, complications.
In this article, we review the incidence, presentation, risk factors, management and preventative strategies of three major complications associated with CB ablation: phrenic nerve injury (PNI), AEF and bronchial injury (Table 1, Table 2).
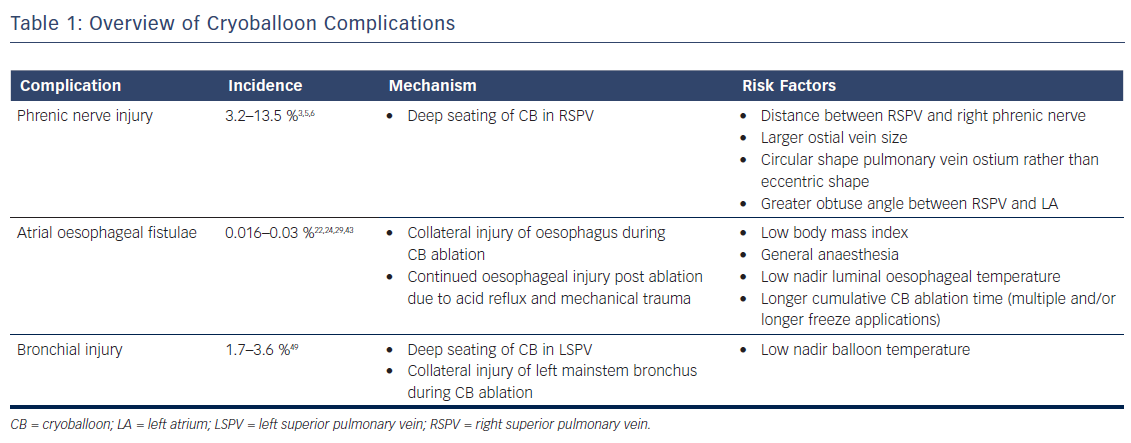
Phrenic Nerve Injury
The incidence of PNI with CB ablation has decreased over the years, probably resulting from increased operator experience and improved techniques for early detection. In the STOP-AF trial, a randomised trial comparing drug therapy versus CB ablation utilising the first generation CB (Arctic FrontTM, Medtronic), the rate of PNI was noted to be 13.5 %.5 In Freeze AF, a randomised control trial comparing first generation CB ablation versus radiofrequency ablation, 5.8 % of patients in the CB arm were noted to have phrenic nerve palsy and zero patients in the RF arm. In a single centre study of 500 consecutive patients from 2012 to 2015 who underwent CB ablation using the second generation CB, PNI was noted in 7.2 % of patients.6 In more recently published data utilising contemporary techniques, rates of PNI appear to be lower. In the landmark FIRE AND ICE trial, in which both first and second generation CB (Arctic Front AdvanceTM, Medtronic) were used, 3.2 % of patients experienced PNI.3
The anatomical relationship between the phrenic nerves and pulmonary vein anatomy is fundamental to pathogenesis of PNI. The left phrenic nerve courses anteriorly across the left atrial appendage during its descent to the diaphragm.7 The right phrenic nerve descends parallel along the anterolateral aspect of the superior vena cava, and then courses posteriorly in between the right pulmonary veins and the superior vena cava–right atrial junction. Histological studies have shown that there may be as few as 2.1 mm (±0.4) and 7.8 mm (±1.2) of distance between the right phrenic nerve and the anterior wall of the right superior pulmonary vein and the right inferior pulmonary vein, respectively.8 This close proximity accounts for why the PNI risk is highest during ablation of the right superior pulmonary vein. However, there are case reports of left PNI during CB ablation of the left superior pulmonary vein.9 Multiple risk factors have been associated with the development of PNI. The common theme among these risk factors is inadvertent deep seating of the CB, which has been shown to distort pulmonary vein anatomy leading to an even shorter distance between the vein and the phrenic nerve.10 These risk factors include:
- the distance between the right superior pulmonary vein and right phrenic nerve as assessed on pre-operative imaging;11
- larger ostial vein size;12,13
- a more circular shape pulmonary vein ostium rather than eccentric shaped ostium;12 and
- an obtuse angle between the right superior pulmonary vein and left atrium (i.e. minimal angulation of the vein and left atrium).13
A vein with a large, circular-shaped ostium with an obtuse take off can inadvertently lead to a more deeply seated CB, and thus higher rates of PNI. Care should be taken to avoid deep seating of the CB by noting the position of the balloon relative to the cardiac silhouette – a greater portion of the CB lying outside the cardiac shadow has been associated with higher rates of PNI.14
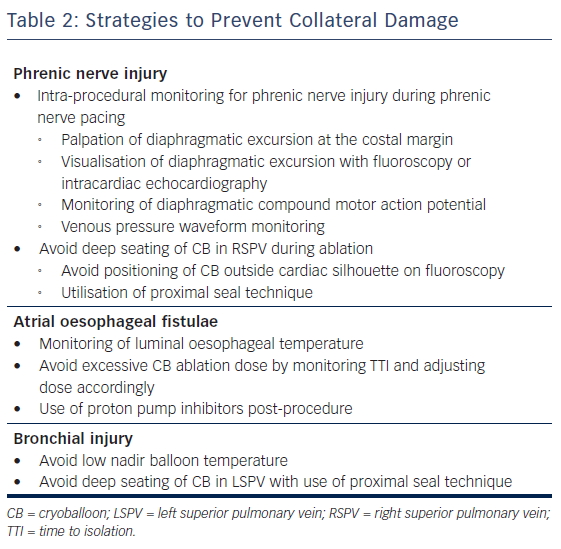
Multiple methods for phrenic nerve monitoring during CB ablation have been published.15–19 It is imperative that the patient not be under any paralytics during this portion of CB ablation. All methods use a pacing catheter to capture the phrenic nerve cranial to the site of CB ablation. Although any catheter can be used, our institution uses a deflectable multipolar catheter placed at the right brachiocephalic vein and superior vena cava junction paced at a cycle length between 1 and 2 seconds at twice the capture threshold. It is important to maintain stable catheter position since loss of capture can be mistaken as PNI during CB ablation.
During phrenic pacing, various techniques have been described to assess for impending PNI. These can be classified as a qualitative or quantitative methods. The most common qualitative method used is palpation of diaphragmatic excursion at the costal margin, with either diminishment in strength or loss of excursion considered of concern for PNI. Visualisation of diaphragmatic excursion with either fluoroscopy or intracardiac echocardiography has also been described.16 The most well described quantitative method for assessment of PNI is the monitoring of diaphragmatic compound motor action potential (CMAP), in which diminishment in signal amplitude of CMAP considered of concern for PNI. The diaphragmatic CMAP can be recorded using a modified surface lead I17 or a quadrapolar catheter in the hepatic vein.18 Another objective method for phrenic nerve monitoring is the use of venous pressure waveform monitoring, in which a venous sheath is attached to a pressure transducer;19 in this case, reduction in pressure signal amplitude is considered of concern for PNI. Although there are no prospective randomised control trials comparing the effectiveness of strategies, most experts agree that at least two methods should be used for phrenic nerve monitoring.15
If PNI is suspected, an immediate balloon deflation, rather than merely stopping refrigerant flow to the balloon, is the recommended course of action.20 If PNI persists at the end of the case, the diagnosis is confirmed by the visualisation of an elevated hemidiaphragm on inhalation/exhalation chest X-ray. The overall prognosis for this condition is good – a majority of the patients have phrenic nerve recovery within 1 year. In a post-approval study of patients enrolled in the STOP-AF trial, fewer than 1 % of patients had persistent PNI after 12 months.21
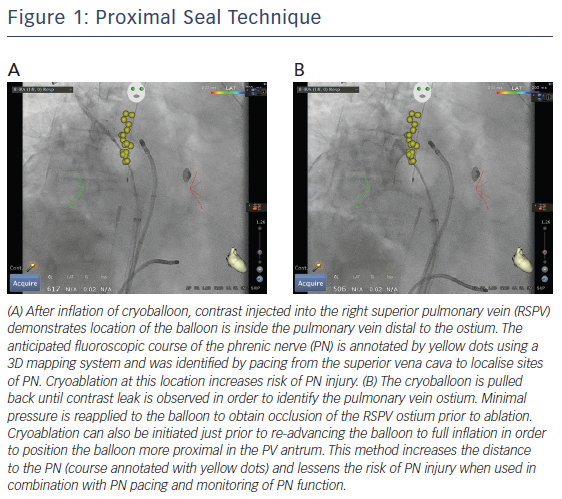
The use of a ‘proximal seal technique’ to increase the distance between the phrenic nerve and the CB may reduce the likelihood of PNI.22 Proximal seal is achieved by first injecting the contrast during the occlusion (Figure 1A). If good occlusion is observed, one should not immediately initiate ablation, but slowly relax the sheath or withdraw the catheter and allow the CB to retract until contrast leaks around the true pulmonary vein ostium (Figure 1B). Often, a large right superior pulmonary vein may have a deep engagement with the CB and the initial occlusion without balloon deformation may appear ostial; however, the CB may be a few centimetres deeper than intended. Only apply the minimal amount of pressure to regain occlusion before ablation. This will also likely reduce the risk of collateral injury and ablate in the true antrum of the pulmonary vein. Multicentre experience of CB ablation using the proximal seal method combined with time-to-isolation dosing has reduced the incidence of PNI to 0.34 % when performed with monitoring of phrenic nerve function.23
Atrial Oesophageal Fistulae
AEF is a deadly complication of CB ablation; the mortality rate is well over 80 %.24 Patients with AEF may present with something relatively benign, like odynophagia, or more serious clinical conditions, such as gastrointestinal bleeding, endocarditis, severe sepsis or cerebrovascular accident.25–27 Stroke, secondary to air or food embolism, is the most common clinical presentation among patients with AEF.24 Given the variety of clinical presentations, a high index of suspicion after recent AF ablation is needed to make a diagnosis.
The pathogenesis of AEF is related to the close proximity of the oesophagus to the posterior wall of the left atrium. However, the precise mechanism is unclear. A proposed hypothesis is that oesophageal injury during AF ablation is the triggering event, with progressive inflammation and injury resulting from swallowing and gastric reflux, leading to continued oesophageal injury, perforation, and fistulae formation.28 The theory of oesophageal injury leading to AEF, rather than left atrial perforation, is consistent with the delayed time course of the disease. Patients appear to present approximately 19 days after ablation, with some patients presenting as early as 6 days and as late as 59 days.29
Fortunately, the incidence of AEF is quiet low. In a survey of 405 physicians from 2013 to 2014 who had performed a total of 191,215 AF ablations, 31 cases of AEF were reported – a rate of 0.016 %.29 An older physician survey notes a slightly higher rate of AEF. In a survey of 585 physicians from 2004 to 2005 who had performed 20,425 AF ablations, six cases of AEF were reported – a rate of 0.03 %.24 Of note, these rates of AEF are largely with RF ablation. The risk of AEF formation with CB ablation is unknown but much lower, and an AEF after CB ablation is a case-reportable event.30–33 Some authors estimate that although the risk of AEF with RF ablation is between 1:400 and 1:1000, the risk with CB ablation is 1:10,000.22
Despite the low incidence, given the high mortality associated with this condition, strategies to identify high-risk patients and techniques to avoid this complication are warranted. Although much of the research has been in patients who have undergone RF ablation, some of the results can be extrapolated to the CB ablation patient. In a study of 104 patients who underwent RF ablation followed by upper endoscopy within 48 hours, 9.6 % of patients were found to have asymptomatic oesophageal injury. On multiple regression analysis, the only risk factor that was significant for oesophageal injury was low body mass index.34 Another risk factor appears to be the use of general anaesthesia. In a study of 50 patients undergoing RF ablation in which patients were randomised to general anaesthesia versus conscious sedation, the rate of oesophageal injury as assessed by capsule endoscopy was 48 % in the general anaesthesia arm and only 4 % in the conscious sedation arm.35 The authors attributed this finding to reduced motility and peristalsis during general anaesthesia, exposing the same oesophageal tissue to RF energy.
The most common intra-procedural strategy to minimise oesophageal injury is the use of luminal oesophageal temperature (LET) monitoring, with cessation of ablation when oesophageal temperature becomes too low. However, there is no consensus of what nadir oesophageal temperature should be used as a safety cut-off. In a study of 32 patients who underwent CB ablation with the second generation catheter utilising two 240 seconds applications for each vein, the authors noted that a minimal LET of ≤12°C was associated with higher rates of oesophageal injury as assessed by postoperative upper endoscopy.36 In a more recently published study with a larger study cohort (92 patients), the same authors found that a higher cut-off of 15°C further reduced oesophageal injury.37 Another study utilising deflectable and non-deflectable temperature probes and a single 3-minute freeze with the second generation CB found a similar temperature cut-off (12.8°C) to decrease risk of oesophageal injury.38 However, one study found an even lower temperature cut-off of 10°C utilising the second generation CB and two 240 s freezes.39 It is worth noting that in all these studies, the oesophageal lesions were healing on follow-up endoscopy, and no patients developed AEF. It is thought these oesophageal ulcers or injuries are precursors to AEF. Although further studies may help to help establish a safety cut-off LET, it is important to emphasise that oesophageal ulceration or AEF may occur without a measured drop in LET.40 We can only say that when the oesophageal temperature is low, oesophageal ulceration or injury is more likely to occur. However, when the measured oesophageal temperature is not low, it does not exclude the possibility of AEF formation.
Interestingly, the left inferior pulmonary vein appears to be the most common culprit in AEF formation after CB ablation. In a recently published case control study in which the authors obtained 11 AEF cases from the Manufacturer and User Facility Device Experience database, 10 of the cases of AEF involved the left pulmonary veins, and eight of the 10 involved the left inferior pulmonary vein.41 The left inferior pulmonary vein often requires more posteriorly directed forces to achieve isolation. These posteriorly directed forces shorten the left atrium-to-oesophagus distance, which has been found to be a risk factor in the development of oesophageal injury.42 Given these findings, proceduralists should practice extra precaution when ablating the left inferior pulmonary vein.
The incidence of AEF is also related to the freezing time or cryoablation dose. The initial clinical trials used traditional 240 seconds freeze–thaw–freeze (two cycles per pulmonary vein at the same location) ablation for the first generation balloon. When the same protocol was used for the second generation CB, the highest incidence of AEF (7/30,000 reported cases) was observed.43 After reducing the standard dosing to 180 seconds, which was based on consensus user experience, AEF is now rarely observed (1/120,000 reported cases) when using two conventional applications per pulmonary vein.43 However, multiple consecutive applications, longer cumulative CB ablation time and higher ‘dosing’ for a single pulmonary vein are still considered incremental risk factors for collateral injury, including AEF.40
Some studies suggest adjusting CB ablation dose based on the time-to-isolation (TTI) of the pulmonary vein may reduce collateral injury or AEF. Observing the physiology of pulmonary vein isolation using an intraluminal circular mapping catheter (AchieveTM, Medtronic) positioned between the left atrium and proximal pulmonary vein during CB ablation may be the most important factor in determining the permanence of pulmonary vein isolation. To better observe the pulmonary vein electrogram of interest, the circular AchieveTM can be positioned in the ostium of the pulmonary vein or prolapsed in front of the inflated CB to assess TTI. Predictors of permanent pulmonary vein isolation are a TTI of less than 30 to 60 seconds. If a rapid TTI is seen with less than 30 seconds, the total ablation time may be reduced to 150 seconds.22
Experimental canine data have shown that permanent pulmonary vein isolation can be reproduced with a TTI of 20 seconds, with the total ablation time of only 80 seconds.44 Conversely, a long TTI of greater than 90 seconds with an additional 180-second freeze is generally not able to permanently isolate the vein, even though initial acute isolation was seen. Longer CB application time beyond 180 seconds will create a deeper lesion and increase the risk of collateral injury or AEF, but not improve pulmonary vein isolation efficacy. Therefore, we do not recommend increasing ablation time when acute isolation does not occur with the first CB application. If TTI does not occur within 90 seconds, we recommend stopping the application and repositioning the CB catheter to modify tissue contact, confirm pulmonary vein occlusion before freezing and aim for TTI < 60 seconds to achieve permanent pulmonary vein isolation.22,44 Reducing application time during a repeat freeze to 150 seconds or less may be warranted due to faster temperature decline on the repeat freeze, which may also avoid deep tissue injury. Repeated ablation more than twice at the identical location or same CB catheter position should be avoided to prevent collateral injury.
Although there are no clinical data to support the use of proton pump inhibitors (PPIs) after AF ablation, most physicians prescribe a short course of these medications to reduce AEF risk.45 Additionally, in the studies entailing endoscopic evaluations after CB ablation, most practitioners prescribed PPIs, and all patients with oesophageal injury were noted to have healing or healed lesions on follow-up.36–38 It is a routine practice in our institution to prescribe 4 weeks of PPI since there are only minimal side effects of a short course of PPI, and we believe that the benefits outweigh the risks.
Patients with suspected AEF require rapid evaluation and treatment. Given the lack of specific findings, a high index of suspicion is required to make a diagnosis. An upper endoscopy is absolutely contraindicated given the risk of massive air embolism after air insufflation during endoscopy.27,45 Either computed tomography (CT) or magnetic resonance imaging (MRI) of the chest is needed to make the diagnosis when air is found in the mediastinum, pericardium or left atrium.26,27 While oesophageal stenting is a therapeutic option, surgery offers the best chance of survival.26,46–48
In addition to AEF, CB ablation has been associated with various upper gastrointestinal dysmotility syndromes, including gastroesophageal reflux and gastroparesis.49 The hypothesised mechanism is injury to the parasympathetic nerve inputs that overly the oesophagus during ablation. The exact incidence of these injuries is unknown because symptoms may be subclinical and practitioners may not have the necessary index of suspicion to make the diagnosis. In small observational studies, the incidence of gastroparesis in patients undergoing CB ablation was approximately 9–17 %.50–52 In one study of 66 consecutive patients undergoing CB ablation, all six patients were asymptomatic and the diagnosis was made on post-procedure upper endoscopy as part of the study protocol.51 A larger study of 144 patients who underwent upper endoscopy as per study protocol found 18 patients with gastric hypomotility, all of whom were asymptomatic.52 Lastly, in a study of 58 patients undergoing CB ablation, the six patients who reported gastrointestinal symptoms intra-procedure had prospective evaluation to make the diagnosis and all six patients had symptomatic recovery within 2 months.50 Further large-scale studies are warranted to identify the true incidence, develop strategies to minimise collateral risk and examine the long-term prognosis of these injuries.
Bronchial Injury
Bronchial erosion and injury is becoming recognised as another serious complication of CB ablation. Although the exact incidence is not known, small single-centre studies have reported rates of 1.7 % and 3.6 %.53 Patients can present with a cough and production of blood tinged sputum54,55 or frank haemoptysis.56–58 Onset of symptoms can be variable. Patients may become symptomatic during the procedure58 on postoperative Day 154,55,57 or even a day later.56,57,59
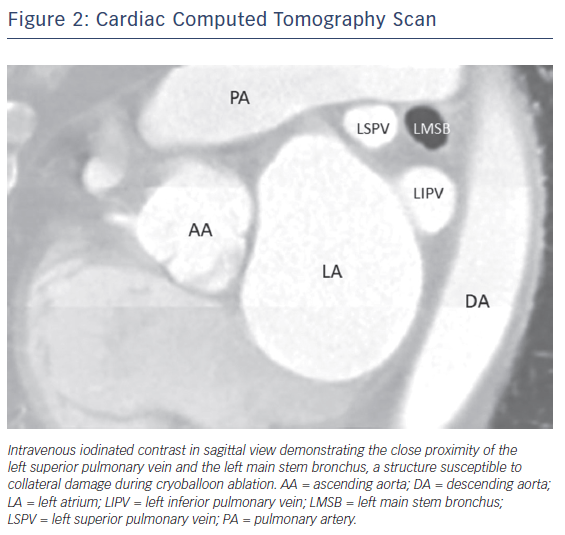
Although the exact mechanism of injury is not known, case reports suggest that these injuries result from extremely low nadir temperatures (–60°C or lower) and deep seating of the CB54,56 leading to collateral thermal injury of the bronchial tree. Radiological studies have shown the close proximity of the bronchial system to the pulmonary veins, making this structure prone to injury during CB ablation (Figure 2).60,61 A recently published case series of CB ablation patients who underwent real-time bronchoscopy found ice formation in the left main stem bronchus in 70 % of patients during ablation of the left superior pulmonary vein (average nadir CB temperature of –48.5°C).62 These findings of thermal injury have been replicated in pig studies, where lower nadir temperature during CB application (–66°C vs –45°C) showed increased bronchial mucosal oedema, erythema and inflammation in post-ablation bronchoscopy.63
No definitive recommendations exist for the management of bronchial injury. Protocol at our institution for suspected bronchial injury entails anticoagulation reversal with protamine, urgent bronchoscopy and, at times CT of the chest to evaluate for consolidation suggestive of pulmonary haemorrhage. Bronchoscopy findings can range from mucosal petechiae,54 bronchial wall erosion56 to haematoma formation.55 Lastly, cough suppressants may play a role in the management of bronchial injury.55 Although the prognosis is not precisely known given limited data, on the basis of case reports it appears that most patients do well on follow-up.54–57 However, rare catastrophic cases of atriobronchial fistula have been reported.59,64
Conclusion
Cryoballoon ablation has become a widely accepted tool for the management of atrial fibrillation. However, cryoballoon ablation can lead to collateral extra-cardiac damage, which can lead to catastrophic complications: phrenic nerve injury, atrial oesophageal fistula and bronchial injury. Although these complications are rare, electrophysiologists should institute measures to identify high-risk patients, implement best-practice techniques to minimise risks and maintain a high index of suspicion to recognise the complications quickly and implement correct treatment strategies.
Clinical Perspective
- Cryoballoon catheter ablation to achieve pulmonary vein isolation has emerged as a major technique for the treatment of atrial fibrillation.
- Atrio-oesophageal fistula, bronchial injury and phrenic nerve injury are rare but serious complications associated with cryoballoon ablation.
- Knowledge of techniques for cryoballoon catheter positioning, methods for monitoring collateral tissue injury and dosing of cryoablation time may reduce the three major complications.