There was a joke years ago that no one understood electrophysiologists, or even wanted to, and that suited us just fine. We were happy to be isolated in our labs working on largely academic problems. Fast forward to now, and the monumental advances in our ability to map and ablate arrhythmias and in new strategies for implantable device therapy have thrust us into the realm of high-volume interventional operators. Our enthusiasm for providing a procedural answer to our patients has not always been accepted by our referring colleagues. In truth there is often great reluctance from many cardiologists to refer patients for ablation. The Catheter Ablation versus Anti-arrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial, although enormously valuable, brought a firestorm of criticism.1,2 In fact, this scepticism is healthy and requires us as a specialty to look more critically at the value of our interventions. Much like the emerging question of the value of percutaneous coronary intervention (PCI) compared with optimised medical therapy for stable coronary heart disease,3,4 it is fair to ask whether we are helping patients with procedural interventions, particularly early in the course of disease. This inability to make our point convincingly has created in effect an ‘identity crisis’ that has many contributing factors. In this article, we explore several themes related to clinical trial interpretation that complicate the ability of cardiologists and patients to understand the impact of our interventions. We hypothesise that our difficulties in performing large randomised clinical trials and our loose interpretations of observational studies is a large part of the problem; in addition, it is the only part that we completely control.
The Curse of the Kaplan–Meier Survival Curve
Not to single out a specific trial but consider the Catheter Ablation of Stable Ventricular Tachycardia before Defibrillator Implantation in Patients with Coronary Heart Disease (VTACH) trial.5 One hundred and seven patients with healed infarction, reduced left ventricular ejection fraction (LVEF; ≤50%) and stable ventricular tachycardia (VT) were randomised to catheter ablation or not prior to ICD implantation. The primary endpoint was time to first recurrence of VT or VF. At its inception, this was no doubt viewed as the ‘lowest hanging fruit’ for ablation. We understand much more about VT in healed infarction than other clinical situations, and hemodynamically tolerated VT allows precise mapping and elegant ablation. In fact, the trial was designed with the expectation that VT/VF recurrence would occur in 20% of the ablation group and in 50% of controls at 2 years. Mean follow-up was 22.5 months. The primary outcome occurred after a median of 18.6 months in the ablation group and of 5.9 months in the control group (p=0.045; Figure 1). VT recurrence was observed in 51% and in 76% of the ablation and control groups, respectively, despite use of amiodarone in 35% of patients. Furthermore, in an exploratory analysis examining the effect of LVEF, the advantage from ablation was erased in patients with EF ≤30%. Although a positive trial, many were astonished at the unexpectedly high rate of recurrence in the ablation group. As is even more apparent in trials of AF ablation, our expectations are often falsely optimistic when based on the results of uncontrolled, single-centre observational trials; prospective randomised trials are often sobering by comparison.
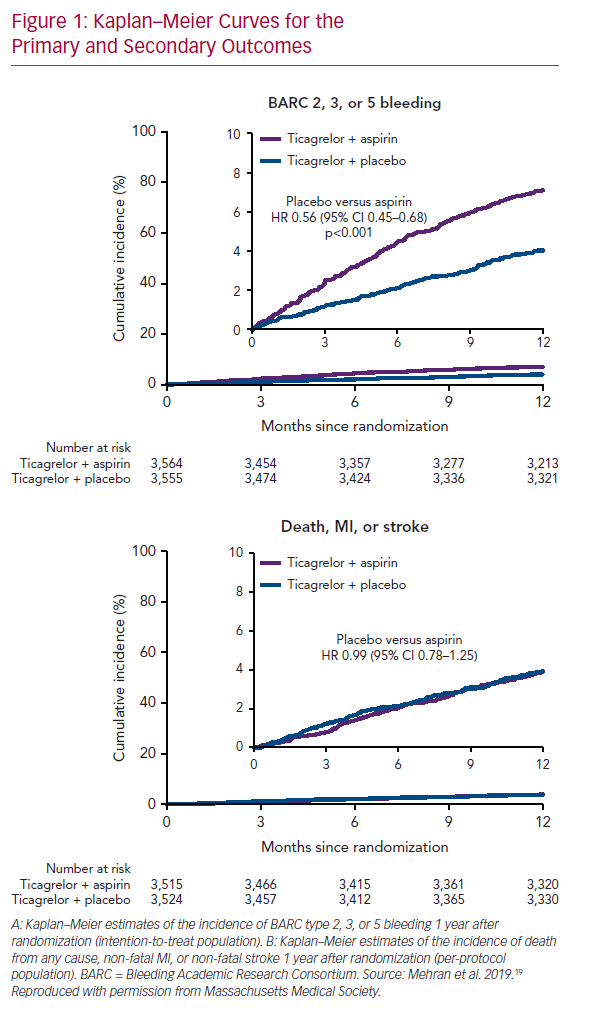
The Kaplan–Meier survival curve is the preferred statistical method for analysing time-to-event data, in large part because of its ability to handle censoring due to subject withdrawal, competing risks (e.g. death) and other causes of uneven follow-up duration. Despite these advantages, the Kaplan–Meier method imposes a binary definition of success/failure, which makes perfect sense in survival trials, but may not be pertinent (particularly to patients) when measuring time to first recurrent arrhythmia; an outcome that may be more meaningful is VT/VF burden before and after intervention. This is more difficult statistically (particularly in AF, where event counting is less complete except in subjects with implanted devices), but probably more applicable from a patient point of view. Despite this demonstrated failure of catheter ablation in the VTACH trial, the number of appropriate ICD shocks was 0.6/year in the ablation group versus 2.4/year in the control group (p=0.015). For most patients, avoidance of ICD shocks is a major goal. A similar outcome is demonstrated in the Kaplan–Meier versus burden analysis of the Substrate Modification Study (SMS).6 The meaningful impact of this intervention is exceptionally difficult to reconcile with what happens in real-world analyses. In one observational trial of patients with VT in the setting of healed infarction, patients were referred only after suffering through a mean of 5.8 ICD shocks in the preceding month: 58% were in VT storm and 68% were prescribed more than 400 mg of amiodarone daily at the time of referral.7
A similar theme is present in trials of AF ablation, but with two additional considerations. The first is the difficulty imposed by guideline-directed assessment of recurrence defined as ≥30 seconds of AF. In the Substrate and Trigger Ablation for Reduction of Atrial Fibrillation (STAR AF II) Trial 589 patients with persistent AF were randomised to pulmonary vein isolation (PVI) alone, PVI plus ablation of complex fractionated electrograms or PVI plus linear ablation (roof and mitral lines).8 Freedom from documented recurrence of AF (≥30 s) on Kaplan–Meier analysis was 59%, 49% and 46% in the three groups, respectively. However, AF burden in all three groups was substantially reduced, from >80% to approximately 5% at 18 months (Atul Verma, unpublished obseration, 2019). Similarly, a very recent randomised trial comparing cryoballoon ablation (with two dosing strategies) with contact force-guided radiofrequency (RF) ablation in 348 patients with paroxysmal AF evaluated freedom from AT/AF following a 3-month blanking period using implantable monitors.9 Freedom from AT/AF recurrence was a rather sobering 53.9% and 52.2% (in the two cryoballoon groups) and 51.7% (in the RF group). However, the AF burden in all groups was reduced by >98%.
The second new consideration is that many of the benefits of ablation are not dependent on elimination of AF but are earned with reduction in burden. The Catheter Ablation versus Standard Treatment in Patients with Left Ventricular Dysfunction and Atrial Fibrillation (CASTLE-AF) Trial demonstrated that catheter ablation, even without total elimination of AF, significantly reduced the primary endpoint of death or heart failure (HF) hospitalisation (28.5% versus 44.6%, p=0.007).10 Although the mean AF burden after ablation varied between 20 and 30% in the ablation group (as compared with 50–60% in the control group), clinically important outcomes were improved.
Our Obsession with Mortality Benefit in Ventricular Tachycardia Ablation Trials
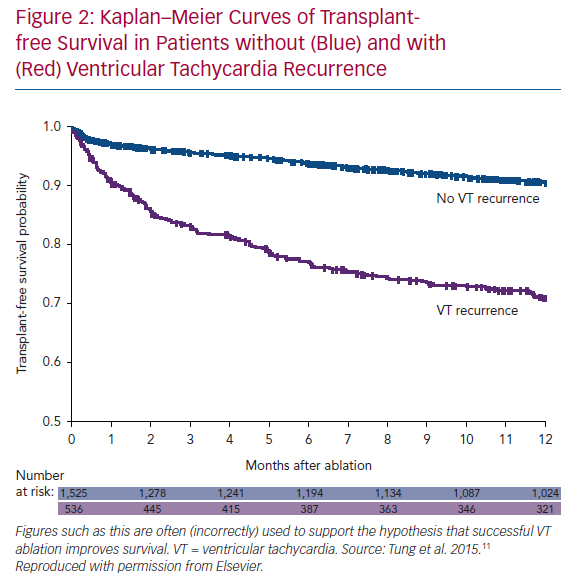
Small randomised trials have not demonstrated a mortality benefit to VT ablation. We seem to expect this, given that successful ablation reduces/eliminates ICD shocks and potentially harmful anti-arrhythmic medications. We have even processed observational trial data to arrive at this conclusion, albeit falsely. The International VT Collaborative Center Study Group provided retrospective, observational data on 2,081 patients with VT in the setting of structural heart disease treated with ablation.11 As seen in Figure 2, patients without VT recurrence had a significantly higher transplant-free survival that those with VT recurrence. This is often touted as a demonstration that VT ablation improves survival, but this is incorrect. The implication, apparently, is that if we were better at ablation, all patients would have successful outcomes. The initial logical error is that all patients received ablation (even the ones with recurrent VT). More importantly, the two groups are different: patients with recurrence had lower EF, higher New York Heart Association (NYHA) class, higher incidence of VT storm, and were more likely to have had failure of ≥2 anti-arrhythmic drugs (AADs) prior to ablation than patients without recurrence. In addition, the groups are different because of the healthy responder bias: a relatively well patient is more likely to respond to a therapy than a sicker patient. This is a powerful (and often unconsidered) confounding principle in considering uncontrolled results. Perhaps the best demonstration of this phenomenon was presented by Goldstein et al., using the Cardiac Arrhythmia Survival Trial (CAST I and II) data.12 They hypothesised that patients who had easily suppressed premature ventricular complexes (PVCs; first AAD, first dose) would have better outcomes than patients who required further drug titration/additional drug trials or those who never had PVCs suppressed. Patients with easily suppressed PVCs had less risk of arrhythmic and total mortality in follow-up. This occurred despite a higher likelihood of being treated with active drug therapy (50% versus 35%), which was shown to cause harm in the randomised trial. Ease of arrhythmia suppression remained a significant predictor of arrhythmic deaths even after multivariate analysis. Response to any therapy (even a harmful one!) selects a population of patients that is likely to do relatively well.
Outcome After Ablation is More About the Patient Than the Procedure
It follows from the discussion above that outcome after VT ablation, both in terms of recurrence and survival, may have more to do with patient than procedural characteristics. This would explain the nearly universal association of freedom from VT recurrence and survival, but not in the way it is usually put forward. Muser et al. examined the performance of eight (largely HF) prognostic risk scores applied to 282 consecutive patients with non-ischemic cardiomyopathy who underwent catheter ablation for VT.13 After a median follow up of 48 months, 43 patients (15%) died, 24 (9%) underwent heart transplantation, and 58 (21%) had VT recurrence. Of the eight models, two performed particularly well: the Seattle Heart Failure Model; and the Chronic Obstructive Pulmonary Disease, Age >60 years, Ischemic cardiomyopathy, NYHA Functional Class III or IV, EF <25%, Presentation With VT Storm, Diabetes Mellitus (PAINESD) score. Both scores had high predictive values for both VT recurrence and transplant-free survival, strongly suggesting that these two outcomes are associated with patient-related (and not procedure-related) variables.
It is tempting to hypothesise that things would work out differently if we intervened earlier: before the relentless march of progressive HF, probably assisted by ICD shocks and AAD toxicity in our VT patients, before atrial fibrosis and remodelling is too established in our AF patients. Two recent trials examined the effect of early intervention in patients with VT in structural heart disease. The Leipzig VT study was an observational study of 300 patients divided into three groups based on time to referral for VT ablation from the first occurrence of VT: group 1 (n=75), <30 days; group 2 (n=84), 1 month to 1 year; and group 3 (n=141), >1 year.14 The groups were different from each other. Group 1 patients had higher EF, lower VT burden and fewer VT morphologies induced with programmed stimulation. At 2 years, VT recurred in 37.3%, 61.9% and 64.5% in groups 1–3, respectively (p<0.0001). However, no survival benefit was observed between the three groups. On multivariate analysis, recurrent VT was a significant risk for mortality. The Preventative or Deferred Ablation of Ventricular Tachycardia in Patients with Ischemic Cardiomyopathy and Implantable Defibrillator (BERLIN VT) randomised patients with healed infarction, EF 30–50% and sustained VT to ablation before ICD implantation (preventative strategy) or after the third appropriate ICD shock (deferred strategy). The primary outcome was a composite of all-cause death and unplanned hospitalisation for VT or HF. The study was stopped for futility after 394 days and enrolment of 169 patients. Mortality occurred in six versus two patients, hospital admission for HF in eight versus two patients, and hospital admission for VT in 15 versus 21 patients in the preventative and deferred groups, respectively.15 It seems that mortality in patients with advanced structural heart disease treated with ICD therapy is determined by the inexorable progression of HF, not the consequences of ventricular arrhythmias.
Early intervention may make sense in AF, at least in patients who do not have the additive pathophysiologic factors of obesity, sleep apnoea and poorly controlled hypertension that seem to confound our best efforts. This hypothesis was not enthusiastically supported by the Medical Anti-arrhythmic Treatment or Radiofrequency Ablation in Paroxysmal Atrial Fibrillation (MANTRA-PAF) trial, which randomised 246 patients with paroxysmal AF to ablation or first ever exposure to Class IC or III AADs. There was no significant difference in cumulative AF burden or burden measured at 3, 6, 12 or 18 months; at 24 months AF freedom was lower in the ablation group (90th percentile, 9% versus 18%; p=0.007), and more patients in the ablation group were free from any AF (85% versus 71%, p=0.004). This trial was limited by cross-over to ablation in 36% of the anti-arrhythmic group and by a non-standard endpoint for PVI. Mortality was not addressed in that small trial. In relatively young and healthy patients a mortality trial would require a substantial period of post-intervention observation, which is difficult to imagine given current funding structures.
Barriers to a Robust Evidence Base in Electrophysiology
In interventional cardiology, almost every important clinical decision is supported by at least a small amount of high-quality evidence. Seemingly, every stent, antiplatelet drug, or combination of drugs has been the subject of one or more randomised trials. By comparison, the amount of high-quality evidence supporting interventions in cardiac electrophysiology (EP) is limited. Why is this? There are five main reasons: distraction caused by the rapid evolution of technology; underinvestment; lack of consensus on procedural endpoints; lack of consensus on techniques; and a therapeutic bias in favour of ablation that stands in the way of equipoise. Together, these factors have created a scientific culture dominated by small-scale, siloed, observational research and unwillingness to collaboratively advance our field with consensus and prospective trials.
Electrophysiologists are u and new technologies are developed at a frenetic pace. First-in-human experiences with promising new technologies and short-term clinical follow up are embraced by journals and readership alike. Performing such a study is surely faster and easier than designing a hypothesis-driven randomised clinical trial; in addition, the time commitment required comes with the risk that the methods used at the outset become archaic by the time of reporting. Newer is not always better; and these early experiences, although necessary and exciting, seldom contribute to the larger questions that we need to answer.
Generating high-quality clinical evidence is expensive. Unfortunately, the usual sources of funding for clinical research have historically provided limited support for EP topics. Manufacturers of ablation and cardiac implantable electronic device (CIED) technologies have generally taken a bare-minimum approach, investing in the least burdensome studies required by regulators for market entry. For AF ablation, this meant trials of <250 patients randomised to drugs or ablation;16,17 for VT ablation, this meant an observational registry;18 for devices it is the ICD-NCDR. In the US, scientific gaps left by industry and academia have traditionally been addressed by the National Institutes of Health (NIH). However, for all of clinical EP, funding from NIH has been limited to approximately 1–2 significant randomised controlled trials per decade. Conversely, and attesting to the need for continued governmental support, the Canadian Institutes of Health Research, which funded the Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs (VANISH) trial,19 has had more impact in the field of VT ablation than the NIH and industry combined. Part of the issue here is scale: ablation and CIED implants are 1–2 orders of magnitude smaller in volume than PCI at its peak. Times are changing, however, and EP interventions should no longer be flying under the radar.
While lack of funding has limited the size and scope of EP research studies, other barriers are more intrinsic to the EP community itself. As described in the preceding paragraphs, we have struggled to define and agree upon endpoints that matter to patients. In clinical practice, we are strongly (and rightly) influenced by a desire to improve our patient’s symptoms, functioning, and quality of life. Yet, we have limited tools for assessing these important outcomes in research studies, with a few reasonable disease-specific qualities of life scales for AF patients, and none at all for patients with VT. We default to endpoints that are easy and relatively inexpensive to measure (e.g. ICD therapies or time to first symptomatic atrial arrhythmia) rather than choosing more meaningful although challenging ones (e.g. AF burden). Then, when studies are completed, we are disappointed when they do not clearly answer critical questions.
EP research is further hampered by a lack of consensus on procedural techniques. EP is loaded with remarkably talented and creative individuals who have achieved incredible things in the service of patients. Nonetheless, things seem to evolve in EP much like they do in the art world, with the avant-garde promoting one or another new technique, and others rushing to follow, trying things out on patients without truly knowing if they are making a difference. For example, with persistent AF, there is some agreement that PVI alone is insufficient for many patients. There is a long (and growing) list of adjunctive ablation techniques, (all of which received a IIb recommendation in the AF ablation consensus statement20) or new technologies (electroporation, radiotherapy), which are all too often rushed through small observational studies into practice without accurate demonstration of safety or comparative efficacy. In the US, we have five EP-focused specialty journals, all with an impact factor <5, publishing an endless number of small observational studies, some of which are technical marvels, but collectively do not seem to move us forward very quickly.
A final barrier to the execution of high-quality randomised trials in clinical EP is a pervasive bias towards intervention. For many electrophysiologists, managing patients with medication in the office is anathema to the preferred clinical activity of doing procedures in an EP lab. Optimal medical therapy for HF is not pursued according to guidelines prior to primary prevention ICD therapy. This behaviour is not unique to electrophysiologists. A recent registry analysis of guideline-directed pharmacologic treatments for patients with HF with reduced EF demonstrated that medication doses were not titrated in >80% of patients; and at 12 months, <1% of patients were receiving target doses of beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor–neprilysin inhibitors and mineralocorticoid receptor antagonists.21 To many, the benefits of invasive therapies are taken as a given, before careful studies are completed. Randomising patients in clinical trials requires equipoise. Too often in EP, we think we know the answer ahead of time, and if we are too proud to admit that the answer is not known, then we will not randomly allocate patients to alternative treatments to find out. To channel our hero Mark Josephson, we are acting like ablationists and defibrillationists, not physician scientists.
Moving Forward
If we are correct that some of the aforementioned issues serve as major impediments to progress in our field, then perhaps we can begin to work through them. We have little doubt that more investment is needed in EP research, both intellectually and financially. It is not enough simply to meet regulatory requirements to bring products to market. We need to ask and answer questions about clinical strategies with our existing therapies to improve clinical decision-making and outcomes for patients.
Arguably, what we need most is to improve our ability to collaborate. We spend too much time trying to perfect our own techniques, and not enough time cooperating to test hypotheses with uniform approaches and patient-centred outcomes. There are examples to point the way, including the development of expert consensus documents on AF ablation that not only made clinical recommendations, but also suggested approaches for future research.20 We should focus on improving the scientific support of our craft as if our field depended on it, because in the near-term future, it likely will.
Clinical Perspective
- We find ourselves having trouble communicating the benefits of our procedures to referring physicians and patients.
- Some of this is related to the lack of high-quality randomised clinical trials.
- Some of this is related to poor messaging of the meaning of results in observational trials.
- The ethos of the physician scientist in clinical electrophysiology needs to change in order to reestablish the scientific basis for our clinical practice.