Sudden cardiac death (SCD) is usually defined as death due to cardiac causes occurring within 1 hour of the onset of symptoms. Unexplained sudden death occurring in an individual older than 1 year of age is known as 'sudden unexplained death syndrome'. Unexplained sudden death occurring in an individual younger than 1 year of age is known as 'sudden unexplained death in infancy'. SCD with negative pathological and toxicological assessment is termed 'sudden arrhythmic death syndrome'.1
Epidemiology
In 2011, approximately 365,500 people (approximately 0.1 % of the population) experienced emergency medical services-assessed out-of-hospital cardiac arrests in the United States.2,3 Of the 19,300 bystander-witnessed out-of-hospital cardiac arrests that year, 31.4 % of victims survived. The annual incidence of SCD increases as a function of advancing age, being 100-fold less frequent in individuals <30 years of age (0.001 %) than it is in adults >35 years.4,5 There is a similar incidence in Europe, with reports of out-of-hospital cardiac arrest ranging from 0.04 % to 0.1 %.6–8 When the aetiological definition is limited to coronary artery disease (CAD) and its tachyarrhythmic burden, the estimate is <200,000 events per year.9
Approximately 50 % of all cardiac deaths are sudden, and this proportion has remained unchanged despite the overall decrease in cardiovascular mortality in recent decades. The proportion of all natural deaths due to SCD is 13 %; if a definition of 24 hours from onset of symptoms is used, it increases to 18.5 %.9 Analysis of data from the Department of Defense Cardiovascular Death Registry in the United States reveals that the incidence of sudden unexplained death per 100,000 person-years is 1.2 for persons aged 18–35 years and 2.0 for those >35 years of age.10 The numbers are higher in the King County (Washington) Cardiac Arrest Database, being 2.1 for infants of 0–2 years, 0.61 for children aged 3–13 years, 1.44 for young people aged 14–24 years and 4.4 for 25–35-year-olds, respectively.11 In the Oregon Sudden Unexpected Death Study, the annual incidence of sudden cardiac arrest among blacks was more than twofold higher than in whites for both men and women.12 In Denmark, the annual incidence of SCD is 2.3 for people aged 1–35 years and 21.7 for people aged 36–49 years.6 Population movement, and especially major train stations, are associated with a higher risk of SCD.13
The main problem with SCD is that the majority of out-of-hospital sudden cardiac arrests occur among patients in whom cardiac arrest is the first clinical expression of the underlying disease or those in whom disease has previously been identified but classified as low risk.9 There is an inverse relationship between incidence and absolute numbers of events, indicating that a large portion of the total population burden emerges from subgroups with lower risk indexes,9 thus making the identification and prevention of future events particularly difficult.
The incidence of sports-related sudden death from any cause in the general population is 0.5–2.1 per 100,000 per year.8,14–16 Although the vast majority of such cases occur during middle age, they represent a relatively small proportion (5 %) of overall sudden cardiac arrest cases.17 The sports-related sudden death rate is higher in elite athletes, with a reported incidence of 1:8,253 participants per year in the National Collegiate Athletic Association (NCAA). Among NCAA division I male basketball players, the incidence is 1:5,200 per year.16 In other studies, the reported incidence of SCD ranges from 0.24 to 3.8 per 100,000, with higher rates seen in African and Afro-Caribbean athletes.18–20,21
Aetiology
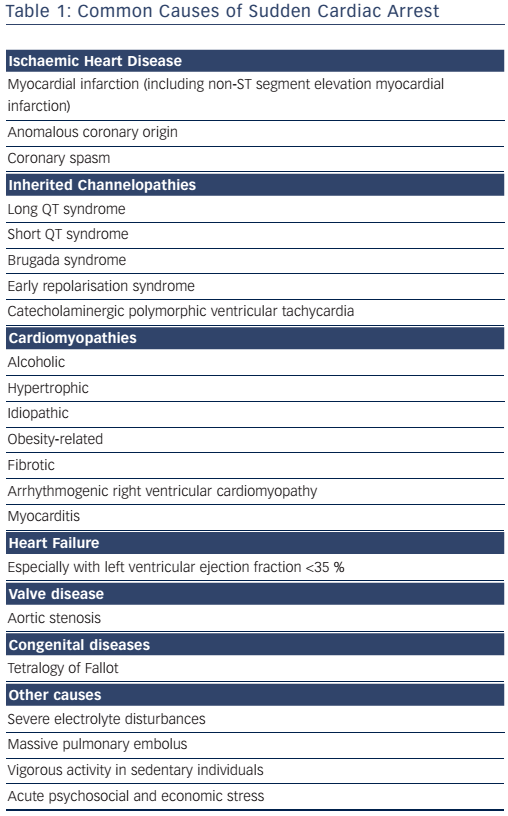
CAD is the most common underlying cause of SCD in the Western world, being responsible for 75–80 % of cases; cardiomyopathies and genetic channelopathies account for most of the remainder (see Table 1).6,22 SCD accounts for 50 % of all CAD-related deaths.9 The incidence of SCD-related atherosclerotic CAD is 0.7 per 100,000 person-years in 18–35-year-olds, increasing to 13.7 per 100,000 in those >35 years of age.10 Female survivors of cardiac arrest are less likely to have underlying CAD (45 %); valve disease and dilated or arrhythmogenic cardiomyopathy are more common.23
Following acute myocardial infarction there is increased risk of SCD during the first months due to tachyarrhythmias or other complications such as re-infarction or myocardial rupture,24 and myocardial scar predisposes to monomorphic ventricular tachycardia (VT). Apart from patients with ST-segment elevation, patients resuscitated from a shockable rhythm should be subjected to coronary angiography.25–27 Although most patients with a cardiac arrest have demonstrable CAD, however, less than half seem to have suffered an acute myocardial infarction.28,29 Only 38 % of cardiac arrest survivors develop evidence of myocardial infarction,22 and the use of tenecteplase during advanced life support for out-of-hospital cardiac arrest does not improve outcomes.30
The most common causes of non-ischaemic SCD are currently cardiomyopathy related to obesity or alcoholism and fibrotic cardiomyopathy.31
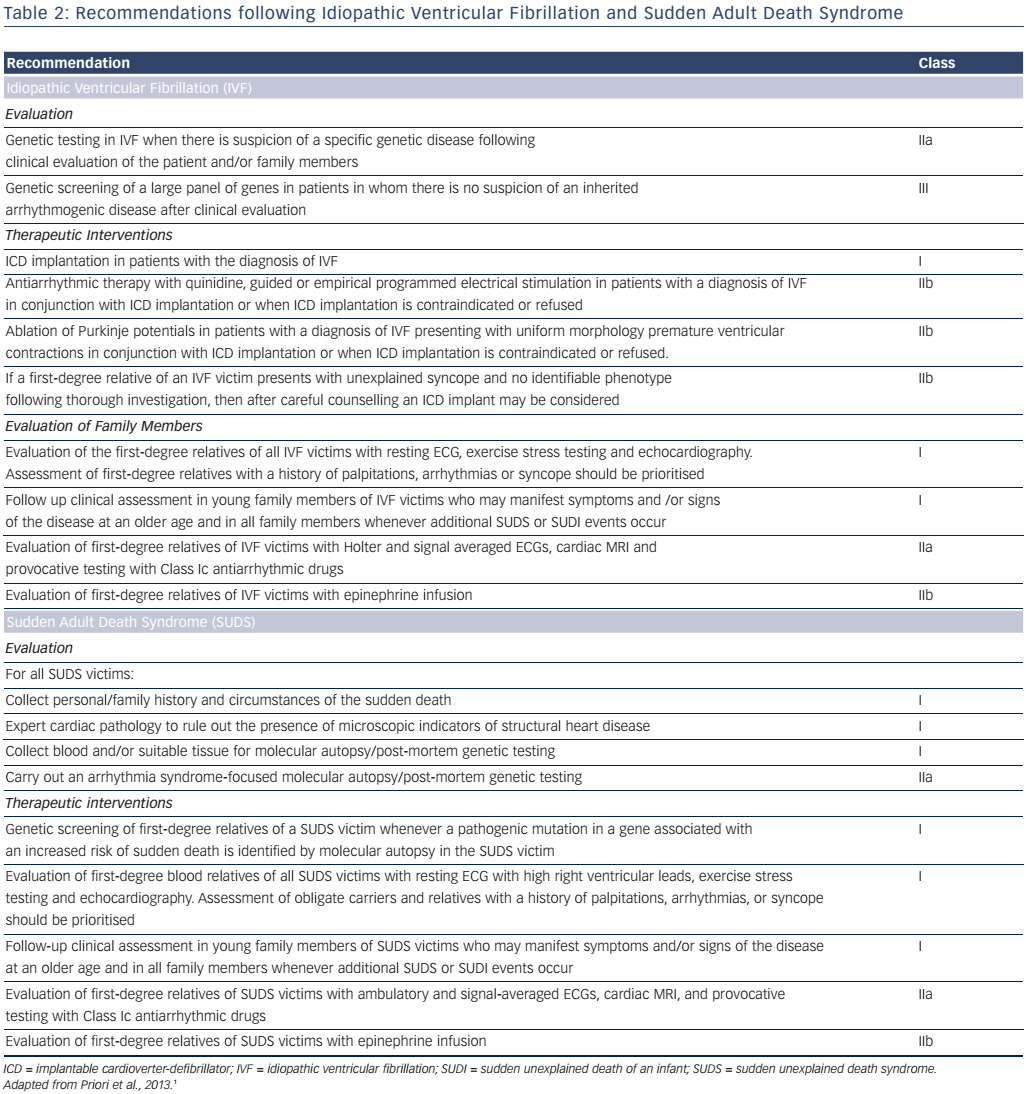
In patients with preserved ejection fraction in the Cardiac Arrest Survivors with Preserved Ejection Fraction Registry (CASPER), an aetiological diagnosis was possible in approximately half of cardiac arrest survivors, the rest being considered cases of idiopathic ventricular fibrillation (VF), presumably due to intrinsic electric abnormalities such as early repolarisation.32 A resuscitated cardiac arrest victim, preferably with documentation of VF, in whom known cardiac, respiratory, metabolic and toxicological aetiologies have been excluded through clinical evaluation is considered to have idiopathic VF.1 Several ion-channel and gene-coding mutations have been associated with idiopathic VF.33 Genetic testing diagnoses an inherited arrhythmia (genetic channelopathy) in up to 29 % of families where a relative has died due to SCD.34 Several studies have also demonstrated a familial predisposition to SCD that may or may not be related to genetic channelopathies.35–37
Coronary spasm is also a cause of cardiac arrest, particularly in male smokers with minimal or no pre-existing CAD.38 Mitral valve prolapse in female patients with ECG repolarisation abnormalities, and frequent complex ventricular ectopy, has also been associated with out-of-hospital cardiac arrest.39,40 An association between air pollution (fine particulate matter with an aerodynamic diameter <2.5 µm and ozone) and out-of-hospital cardiac arrest has recently been demonstrated.41 SCDs account for most cardiac and many non-AIDS-related natural deaths in HIV-infected patients.42 Lower socioeconomic status, depression, anxiety, social isolation and acute emotional stress have all been linked to increased SCD risk.43,44
There is a circadian variation in SCD. The peak incidence of SCD occurs between 6 am and noon (and is blunted by beta-blockers), with a smaller peak occurring in the late afternoon for out-of-hospital VF arrests. The incidence is highest on Mondays.45,46
In the young (<35 years), the most common cause of SCD is arrhythmia, mostly in the context of an apparently normal heart.11,47 The most common causes of SCD are congenital abnormalities in those aged 0–13 years, primary arrhythmia in the 14–24-year age group, and CAD in those >25 years.11 In 5–20 % of cases no significant cardiac abnormality is found at autopsy.10,47 In a recent Danish registry report on individuals aged <50 years, sudden death was caused by noncardiac diseases, such as pulmonary embolism, meningitis and cerebrovascular bleeding, in 28 % of cases.48
In sports-related sudden death in the general population, a clear diagnosis is made in <25 % of cases, but the cause is usually an acute coronary syndrome (75 %).14 In professional athletes, a diagnosis is usually made in up to 65 % of cases and hypertrophic cardiomyopathy (HCM) is considered the main cause, at least in the United States, followed by arrhythmogenic right ventricular cardiomyopathy (ARVC, especially in the Veneto region of Italy), congenital coronary anomalies, genetic channelopathies, myocarditis, Wolff–Parkinson–White syndrome and Marfan syndrome, with blunt trauma, commotio cordis and heat stroke being less frequent causes.19,49,50 There is evidence, however, that HCM may not be the major cause of SCD in athletes.16,20 Autopsies in deceased NCAA athletes most often reveal a structurally normal heart (25 %), followed by coronary artery anomalies (11 %), myocarditis (9 %), ARVC (5 %) and aortic dissection (5 %), with HCM only demonstrated in 8 % of individuals.16 Findings from the Race Associated Cardiac Arrest Event Registry (RACER) indicate that marathons and half marathons are associated with a low overall risk of cardiac arrest or sudden death (1:100,000), with deaths most commonly attributable to HCM (26 %) or atherosclerotic coronary disease (16 %).45 Some of these cardiac arrests might, however, have been provoked by heat stroke.51 CAD is the predominant cause of SCD in older athletes.52 Vigorous exertion can trigger cardiac arrest or SCD, especially in untrained persons, but habitual vigorous exercise diminishes the risk of sudden death during vigorous exertion.53 Most studies have found inverse associations between regular physical activity and SCD.22
Pathophysiology
VT or VF was thought to be the most common cause of out-of-hospital cardiac arrest, accounting for approximately three-quarters of cases,the remaining 25 % being caused by bradyarrhythmias or asystole.54,55 More recent studies, however, suggest that the incidence of VF or pulseless VT as the first recorded rhythm in out-of-hospital cardiac arrest has declined to <30 % in the past few decades.56–58 Pulseless electrical activity (electromechanical dissociation) and asystole are proportionally more frequent mechanisms than VT/VF. Recent data demonstrate a pulseless electrical activity incidence of 19–23 %, with approximately 50 % of patients initially having asystole.59 However, the majority of survivors are in the subgroup whose initial rhythm is VF or pulseless VT.57 VF is a cause of cardiac arrest, and if untreated the arrhythmia is usually fatal. Spontaneous reversions to sinus rhythm have, however, been recorded. Non-arrhythmic mechanisms, such as myocardial rupture or aortic aneurysm rupture, may also result in SCD.
Investigations in Survivors
Overall survival after out-of-hospital cardiac arrest remains low, at approximately 7.6 %,60 but survivors’ quality of life is good for at least for the next 12 months.61 Early coronary angiography in patients resuscitated as a result of a shockable rhythm with immediate percutaneous coronary intervention improves survival.26,27
A full cardiac assessment is needed in cardiac arrest survivors.38 Patients presenting with VF or sustained monomorphic VT are at a considerable risk of recurrence, particularly in the presence of reduced left ventricular function. Studies of out-of-hospital cardiac arrest survivors as well as of patients with sustained VT have shown that the actuarial incidence of sudden death 2 years after the presenting arrhythmia varies from 15 % to 30 %. Up to 74 % of patients with out-of-hospital cardiac arrest have VF recurrence during prehospital care, and the time in VF is associated with a worse outcome.62 Long-term survival among patients who have undergone rapid defibrillation after out-of-hospital cardiac arrest, however, is similar to that among age-, sex- and disease-matched patients who have not had out-of-hospital cardiac arrest, although only 40 % of survivors had an implantable cardioverter defibrillator implanted.63
Family members of young SCD victims are at increased risk for ventricular arrhythmias and ischaemic heart disease. Screening of first-degree relatives, especially those <35 years old, is important.64 When findings suggest cardiomyopathy or genetic channelopathy, the evaluation of other family members is also necessary. The examination of relatives (cascade family screening) may have a significant diagnostic yield.65 It should be noted, however, that no reported history of sudden death among the relatives of most young (<35 years) decedents may be identified.47 Investigation into the genetic basis of SCD, such as the candidate gene approach, has explored the potential association between SCD in CAD and genes associated with genetic channelopathies. Genome-wide association studies are promising but of limited clinical value.9
The following tests are useful for establishing a diagnosis in SCD survivors:38
- ECG (ischaemia, myocardial infarction, inherited channelopathies).
- Echocardiography (heart failure, cardiomyopathies, valve disease, congenital heart disease).
- Coronary angiography (CAD, congenital coronary anomalies, coronary spasm).
- Exercise test (ischaemia, long QT syndrome [LQTS], catecholaminergic polymorphic ventricular tachycardia [CPVT]).
- Electrophysiology testing (induction of arrhythmia, pharmacological provocation for Brugada syndrome, LQTS, CPVT). Procainamide testing may provoke a Brugada pattern irrespective of the baseline ECG and should be considered in the workup of SCD.66
- Cardiac MRI (ARVC, sarcoidosis, myocarditis, myocardial injury from coronary spasm).
- Genetic testing is indicated when an inherited phenotype is detected (ARVC, Brugada syndrome, CPVT, LQTS). Its role in phenotypicallyambiguous or -negative patients is not established, since it is not always possible to differentiate between disease-causing mutations and irrelevant genetic variants. The recommendations for genetic testing are presented in Table 2.
- Cardiac biopsy may also be needed in elusive cases.
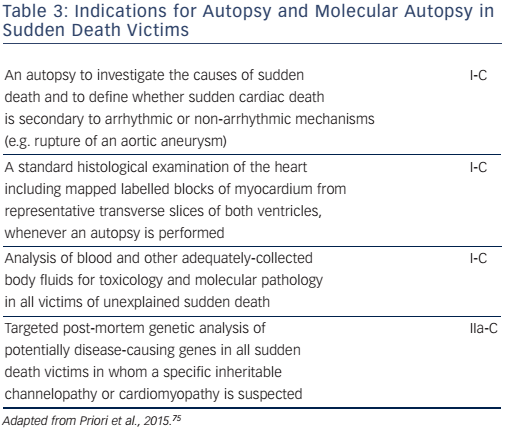
A molecular autopsy may also be considered as part of the community forensic investigation to enhance SCD prevention for other family members (see Table 3). Thus, in cases of documented SCD without an obvious cause, collection of post-mortem blood in ethylenediaminetetraacetic acid, i.e. the purple-top tube to enable DNA extraction, for subsequent DNA analysis may identify a cause of death in up to 30 % of cases.67 A forensic examination, including a toxicology screen, may establish the diagnosis in cases of traumatic, toxic or cardiac causes. A negative pathological examination suggests a genetic channelopathy.68 Post-mortem MRI is also a valuable tool for non-invasively documenting pathological findings, such as myocardial infarction or severe myocardial hypertrophy,69 and post-mortem computed tomography coronary angiography is now possible.70 Despite every effort, however, nearly half of the causes of cardiac arrest will remain unexplained.32
Management of Cardiac Arrest
A population-based cohort study on out-of-hospital cardiac arrest survivors in Ontario, Canada, detected an improved 30-day survival from 9.4 % in 2002 to 13.6 % in 2011.71 Survival is better in places with facilities for bystander resuscitation.15 Defibrillators in public locations (Table 4), such as train stations,13 however, only reduce the incidence of SCD when the local population is trained to use them.72 Recent data from the United States Cardiac Arrest Registry to Enhance Survival (CARES) suggest that rates of survival following out-of-hospital cardiac arrest have improved among sites participating in a performanceimprovement registry.73 Improved survival rates are more prominent in patients aged 18–80 years.74
Clinical Implications
The annual incidence of SCD increases as a function of advancing genetic channelopathies account for most of the remaining cases. In the young (<35 years), the most common cause of SCD is arrhythmias, mostly in the context of an apparently normal heart. In sports-related sudden death in the general population, a clear diagnosis is made in <25 % of cases, but the cause is usually an acute coronary syndrome (75 %). Examination of relatives (cascade family screening) may have a significant diagnostic yield and is strongly recommended.