Prior to the widespread availability of left ventricular assist devices (LVADs), many end-stage heart failure patients were forced to fight over scarce transplant resources or face the reality of impending death. For those considered transplant-ineligible, due to medical or psychosocial factors, symptom palliation and end-of-life care were the only available options. This all changed dramatically following the publication of the landmark Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial,1 a multicentre investigation designed to determine the impact of a pulsatile LVAD compared with optimal pharmacotherapy in a high-risk cohort of patients with advanced disease. Boasting a remarkable 48 % reduction in mortality with LVAD support,1 the results of this study promptly ushered in a historic evolution in mechanical circulatory support. Now, little more than a decade after REMATCH was presented, more than 10,000 patients have been implanted with a durable LVAD.2 Novel design changes have ensued, such that modern devices are substantially smaller and no longer pulsatile. By embracing continuous-flow technology, the field has seen incremental gains in patient survival and quality of life. Currently, over 80 % of patients are alive at one year, while two-year survival following device implantation now exceeds 70 per cent.2
Despite enhanced durability and improved long-term outcomes, the LVAD remains susceptible to complications. While untoward sequelae such as bleeding and thrombosis are being increasingly recognised and reported, the influence of cardiac arrhythmia in individuals with an LVAD cannot be overlooked. Rhythm disturbances are, in fact, some of the most common complications. Recent estimates suggest an incidence of 4.66 events per patient year – a figure that would put arrhythmia behind only haemorrhage and infection in terms of magnitude of risk.3 Of particular concern is the development of ventricular arrhythmias during mechanical support. What were once thought to be well tolerated and of little clinical consequence, have now become the focus of intensified basic and clinical investigation. With this in mind, the following review will highlight the epidemiology, symptomatology, complications and management goals for ventricular arrhythmias in the contemporary LVAD era.
Evolution of Ventricular Assist Device Therapy
From a historical perspective, LVADs have evolved considerably in a relatively short period of time. Initial first-generation LVADs were designed to be volume-displacement pumps. They provided pulsatile flow, as a blood-containing chamber would fill and then empty in concert with the heart’s native contraction. These pumps were relatively large and required a number of moving parts in order to function optimally. As a result, they were difficult to implant in smaller patients and were prone to wear over time. In order to overcome some of these limitations, second generation LVADs employed continuous-flow technology through the use of an axial rotor. These devices were considerably smaller, could be implanted in a broader population of patients, and demonstrated enhanced durability. In addition, systemic pulsatility was abandoned in exchange for continuous ventricular unloading. Newer third-generation devices – designed with either hydrodynamically- or magnetically-elevated rotors – now provide cardiac support to advanced heart failure patients via centrifugal flow. Though they have only recently been introduced into the marketplace, and are currently the subject of ongoing investigation, implant volumes for these novel LVADs have been escalating rapidly. Table 1 highlights some of the important similarities and differences among first- through third-generation devices.
Epidemiology and Aetiology of Ventricular Arrhythmias During LVAD Support
In spite of the technological improvements described previously, device complications still plague LVAD populations. As mentioned, ventricular arrhythmias are among the most common, occurring in 20–50 % of supported patients.4–9 While the greatest risk tends to be in the first month following device implantation,8 there is a growing body of evidence to suggest that later arrhythmic events are still important. In addition, recurrence following an initial episode is common,9 making care of affected patients an ongoing and laborious challenge.
There have been very few baseline patient or device characteristics that have been shown to be associated with the development of ventricular arrhythmias, and most of these associations have been of limited statistical rigor. Some investigators have demonstrated greater rates of arrhythmic events among patients with ischaemic heart disease,10,11 while others have suggested that non-ischaemic patients are at higher risk.8 The absence of postoperative beta-blocker therapy,12 prolongation of the baseline QT interval,13 and deranged serum electrolytes have all been touted as risk factors as well,11 but none of these have been well validated. In fact, only the presence of pre-implantation ventricular tachycardia (VT) has been repeatedly shown to be a powerful predictor of future episodes of VT after LVAD.4,6,8,9
Mechanisms of Ventricular Arrhythmias in the LVAD Patient
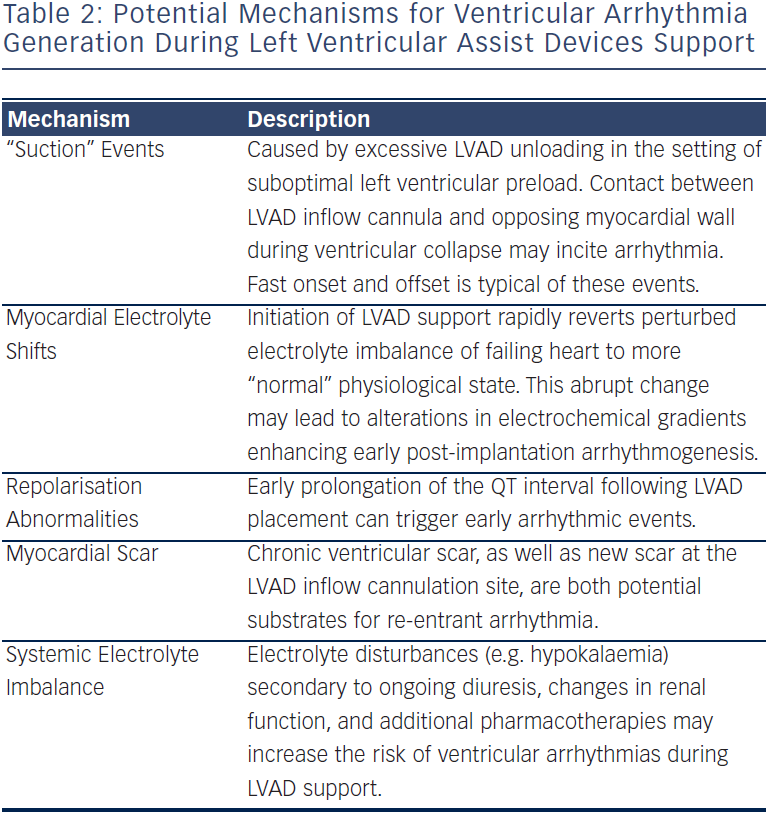
Table 2 lists proposed mechanisms for the provocation of arrhythmias during LVAD support. It is not only plausible, but also quite probable that the majority of these conditions do not exist in isolation, but rather may conspire together to trigger an arrhythmic event. Nonetheless, a careful examination of each of these potential mechanisms should be conducted when considering an optimal therapeutic approach to patient management.
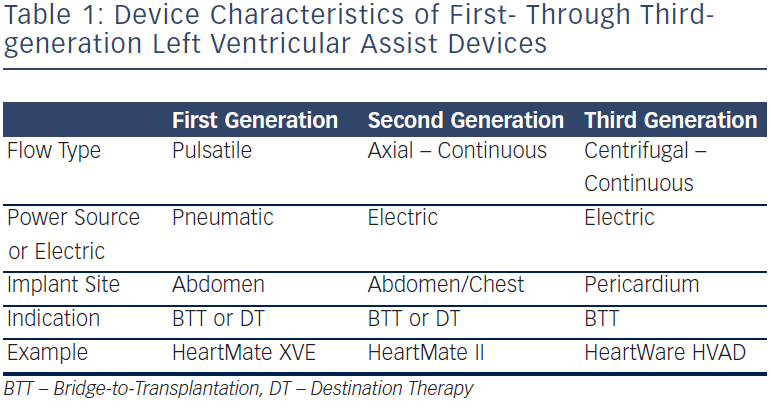
Continuous-flow LVADs, with either axial- or centrifugal-flow designs, maintain constant unloading of the left ventricle throughout the entire cardiac cycle. As a result, these devices are considerably more susceptible to preload and afterload changes than their pulsatile-flow predecessors. Suction events occur when the LVAD unloading exceeds the capacity of the left ventricular preload. In such cases, the ventricular chamber collapses and the inflow cannula can then make direct contact with the opposing ventricular wall, resulting in a so-called “suction” episode (see Figure 1). This most commonly occurs during periods of hypovolaemia, but may complicate any clinical situation resulting in diminished LV filling, including cardiac tamponade, right ventricular failure, and severe pulmonary hypertension. These “suction” events are one proposed mechanism for the development of ventricular arrhythmias, particular the instigation of monomorphic ventricular tachycardia (VT).14 Importantly, the development of suction-related VT is usually considered acute in both onset and offset. Recovery of LV preload, or a reduction in LVAD unloading, can often eradicate these episodes.
Electrolyte shifts associated with device implantation and continuousflow support have also been implicated in the development of ventricular arrhythmias after LVAD. Abnormalities in electrophysiological homeostasis are hallmark characteristics of chronic heart failure.15 While the Na/K-ATPase pump maintains a normal potassium gradient in healthy human hearts, this is not often the case in the failing heart. As a result, myocyte ionic gradients are frequently perturbed, and therefore highly susceptible to sudden depolarisation and arrhythmia formation. At the same time, implantation of an LVAD has been shown to rapidly reverse the electrolyte imbalance of the failing heart, restoring a state of more “normal” physiology. This acute shift in myocardial electrolyte composition, however, has also been shown to enhance arrhythmogenic vulnerability.16
Patients with chronic heart failure also commonly exhibit several characteristic electrophysiological abnormalities including prolongation of the myocyte action potential, increases in the QT interval and diminished heart rate variability.15 While some studies have shown that after sustained LVAD support there is a shortening of the action potential duration and reduction in the QT interval, occurring in parallel with phenotypic ventricular reverse-remodelling, others have demonstrated a paradoxical QT prolongation in the first week following LVAD implantation, and have suggested that these acute repolarisation abnormalities may trigger early ventricular arrhythmias in the LVAD patient.13
Finally, the influence of myocardial scar has also received considerable attention as a substrate for post-device ventricular arrhythmias. The resulting re-entrant VT is a common cause of morbidity and mortality in patients with advanced heart failure, and remains a potent mechanism for VT even after LVAD placement. Additionally, new scar at the apical inflow cannulation site has been shown to be a viable, though less common source of arrhythmia propagation.17
LVAD Outcomes after Ventricular Arrhythmia
Although in the unsupported heart failure patient ventricular arrhythmias can be life threatening, a number of case reports have highlighted their improved tolerability after LVAD implantation. As a result of continuous ventricular unloading, the literature is now replete with cases of LVAD patients maintaining adequate functionality despite weeks or even months of ongoing arrhythmia.18–20 Furthermore, implantable cardioverter defibrillator (ICD) interrogations in LVAD individuals have shown that many patients are experiencing a substantial burden of asymptomatic arrhythmic events.21 Despite these findings, it is anticipated that the majority of individuals will ultimately be affected by their ventricular arrhythmias.9 Most symptoms result from the influence of the arrhythmia on the right ventricle (RV), and the subsequent reduction in right ventricular stroke volume. Manifestations of this can include peripheral oedema, nausea, emesis, hepatic congestion and renal dysfunction, to name a few. Additionally, the reduction in RV stroke volume limits LV filling (and thus LVAD unloading) and can lead to symptoms of low cardiac output, including pre-syncope, syncope, exertional dyspnoea and fatigue.
While it is not clear if the development of ventricular arrhythmias is associated with worsened survival in LVAD cohorts,22 these events do appear to be negatively impacting patient quality-of-life. In a group of patients who routinely value quality over quantity,23 this is an important consideration. Not only are patients challenged physically by the symptoms that may develop, but they are also at much greater risk for re-hospitalisation. In addition, due to the often refractory nature of these rhythm disturbances, management can commonly require multiple pharmacotherapies and even invasive procedures.9
Arrhythmia Management
Several methods for managing ventricular arrhythmias in the LVAD population have been employed with modest success. Medications such as anti-arrhythmic agents and beta-blockers should be utilised when possible in an effort to eliminate the need for more invasive therapies. Both oral and intravenous amiodarone have been previously shown to be the most effective anti-arrhythmic drugs for the secondary prevention of ventricular arrhythmias,9 though the evidence-base supporting this is admittedly thin. Due to the aforementioned refractory nature of many of these events, combination anti-arrhythmic pharmacological strategies are commonly used with varying results.9 In many cases, arrhythmia termination may ultimately require electrical cardioversion. In addition, catheter-based approaches for managing ventricular tachyarrhythmias have been used more frequently. While limited outcome data exist, ablation appears to be safe and feasible.24,25 Though it may not eradicate all rhythm disturbances, this technique seems to reliably reduce arrhythmic burden in the majority of cases. Future prospective study is clearly warranted to address the role of ablation in LVAD arrhythmia management.
Implantable Cardioverter Defibrillators in LVAD Patients
While clearly indicated to reduce sudden death in advanced heart failure populations,26 greater controversy exists regarding the use of ICD therapies in LVAD-supported individuals – particularly those with contemporary continuous-flow devices. Though some investigators have reported survival benefits with ICDs in these patients, and hence advocate ICD implantation in all individuals,6,8 others have shown no demonstrable improvements in mortality.4,21 At this point it is unclear whether everyone with an LVAD should receive an ICD. Given the known adverse impact of ICD shocks on quality of life, the risk of ICD infection and haematogenous seeding of the LVAD pump, and the challenges with electrical interference between some devices, perhaps a more tailored approach to ICD implantation should be considered. In fact, some have advocated the implantation of an ICD only for those with a substantial burden of pre-LVAD arrhythmias.4,8 Additional study will be needed to clarify this ICD debate.
How best to program an ICD during LVAD support also remains uncertain at this time. Given the improved tolerability of ventricular arrhythmias with continuous LVAD unloading, a more restrictive therapeutic strategy is often employed so that only arrhythmic events that are sustained or that result in haemodynamic instability are targeted for ICD intervention. More than likely, novel programming strategies utilising more aggressive anti-tachycardic pacing while limiting ICD discharge will be the goal for future arrhythmia treatment.
Conclusion
The implantation of an LVAD can enhance the functional status and survival of patients with end-stage heart failure. While improvements in pump design have led to greater durability and broader patient applicability, complications of these mechanical devices still exist. Among these are the development of ventricular arrhythmias. Though better tolerated than in unsupported individuals, ventricular arrhythmias can lead to worsening symptoms, increased hospitalisations and diminished quality of life among LVAD patients. Novel strategies for predicting, preventing and treating these common events are needed so that patients may continue to prosper from LVAD support.