AF is the most common clinical arrhythmia that causes severe adverse cardiovascular events, such as ischaemic stroke and acute heart failure.1 Triggers from the pulmonary vein (PV) have been identified as crucial ectopic sources that initiate AF and pulmonary vein isolation (PVI) is the cornerstone for catheter ablation of AF.2,3 Per the European and US AF guidelines, catheter ablation of AF is currently recommended as the first-line therapy if anti-arrhythmic agents fail to maintain sinus rhythm.4–6 The recent advances in mapping and ablation techniques have provided more efficient non-pharmacological therapies for AF. In the Catheter Ablation Versus Anti-arrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial, the catheter ablation group had superior quality of life compared with the anti-arrhythmic drug group and less AF recurrence after blanking through intention-to-treat analysis.7,8 Hence, catheter ablation has become widely used for treating symptomatic drug-refractory AF, even though the recurrence rates of AF ablation remain high, especially in persistent AF and longstanding persistent AF. Therefore, although several multicentre randomised trials showed no difference between PVI alone and additional ablation within the left atrium (LA), various methods, including linear ablation and substrate modification, have been introduced to achieve favourable results.9–12 However, atrial tachycardia (AT) occurring after AF ablation is often symptomatic, complex and poorly controlled by anti-arrhythmic agents.13 Notably, this AT can be classified into the following three categories: focal, macroreentrant and microreentrant AT.14–16 Therefore, the question of how these ATs can be effectively ablated has become a crucial issue in the era of AF ablation. In this review, we summarise the incidence, mechanism, mapping and ablation techniques, and outcomes of AT after AF ablation.
Incidence
The incidence of AT after AF ablation varies from less than 5% to 40% and is associated with the index ablation strategy and duration of AF.15,17–20 ATs after PVI can be due to a focal or macroreentrant mechanism. Karch et al. reported that compared with segmental isolation (2%), circumferential PVI resulted in higher incidence of ATs (18%).21 Focal ATs have frequently been observed from reconnected PVs after a segmental or circumferential PVI and account for up to 80% of AT occurrences,17,22 whereas macroreentrant ATs have been noted after extensive LA ablation.23–25 Linear ablation combined with PVI may result in reentrant ATs because of conduction gaps and non-transmural lesions caused by ablation lesions.26,27 One cohort study conducted in the US demonstrated that ATs after PVI might be single AT or multiple ATs, and nearly 90% were reentrant and associated with gaps in the previous ablation line.28 Chugh et al. reported that nearly 60% of ATs after PVI had critical isthmus that localised to the mitral isthmus.20 Complex atrial fractionated electrogram (EGM)-based ablation is associated with high AT incidence (26–36%).10,29 This indicates that more aggressive and extensive LA ablation lesions might easily produce ATs after the index procedure.
Cryoballoon ablation (CBA) for PVI has safety and efficacy similar to those of radiofrequency catheter ablation (RFCA).30 In one study, the incidence of ATs after CBA was 3–11% and more than half of ATs were macroreentrant.31–36 Chang et al. reported that in the second procedure, higher LA flutter occurred in the CBA group than in the RFCA group (54.5% versus 12.5%).37 The possible explanation is that CBA produced more extensive low LA voltage areas than RFCA, which might have contributed to LA macroreentrant ATs.37 Of all the LA macroreentrant ATs in CBA, perimitral flutter (45.5%) is the most common type, followed by roof flutter (27.3%) and septal flutter (9%).
Surgical AF ablation is an alternative treatment for drug-refractory or even catheter ablation-refractory AF and ATs are often observed after surgical AF ablation. Gopinathannair et al. reported that ATs originated more frequently in the LA (69%) than the right atrium (RA; 31%) and the most common arrhythmia mechanism was reentrant AT (70%). The three most common forms of macroreentrant AT after surgery were cavotricuspid isthmus (CTI)-dependent ATs (24%), perimitral ATs (18%) and roof-dependent ATs (16%).38
Classification and Mechanism
In 2001, experts at the European Society of Cardiology and North American Society of Pacing and Electrophysiology reached a consensus and defined the two typical classifications of AT as focal AT and macroreentrant AT.16 PVI, linear ablation and substrate modification during AF ablation might contribute to the abnormal substrate and possible conduction gaps that could enhance the development of ATs.13
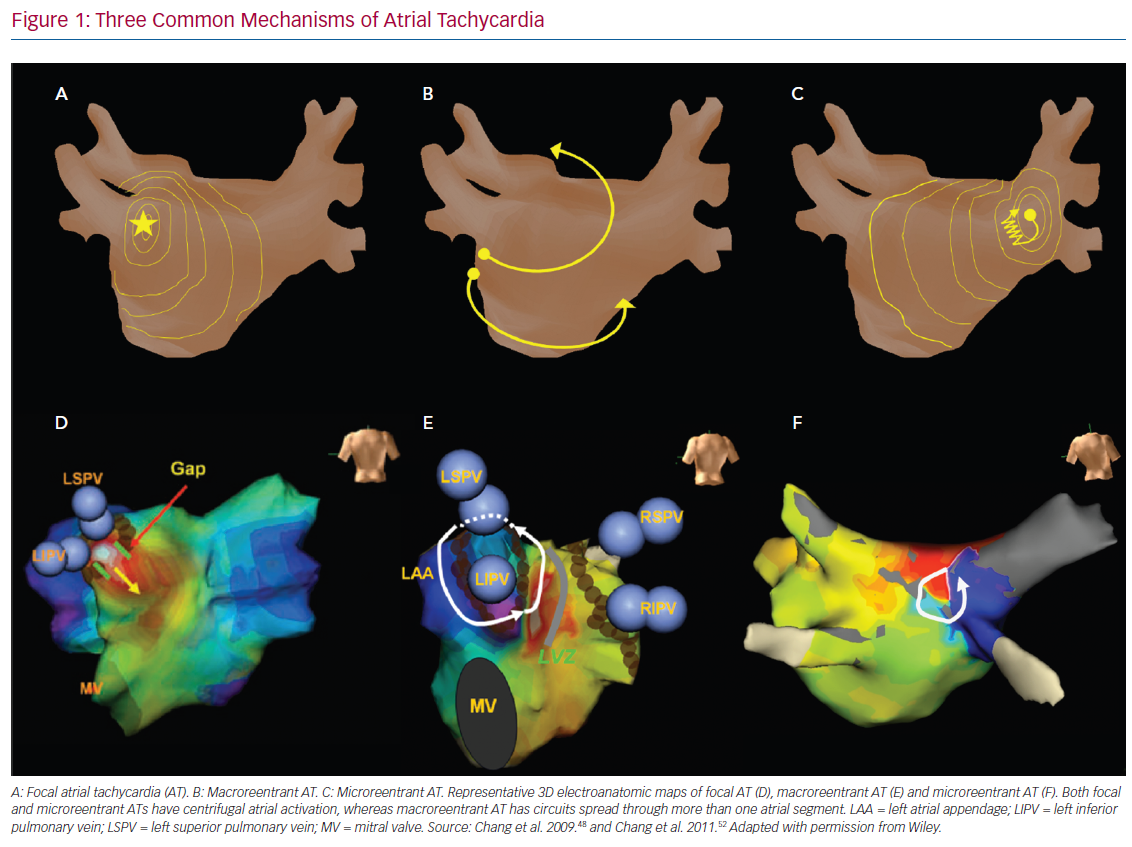
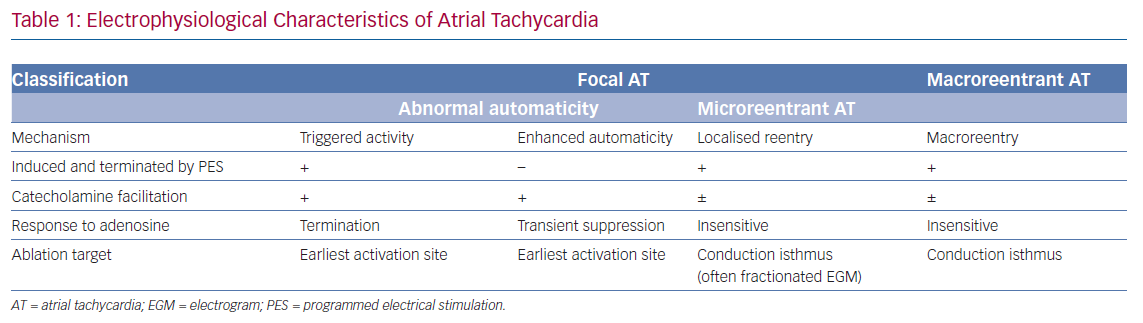
Microreentrant ATs were described later by Jais et al. as a novel mechanism beyond the previous definition of the expert committee.39 These three types of AT possess different electrophysiological characteristics. Comprehensive understanding of the mechanisms of AT after AF ablation could help rapid diagnosis and improve the efficacy and efficiency of catheter ablation (Figure 1). The mechanisms and electrophysiological characteristics are summarised in Table 1.
Atrial Tachycardias Caused by Focal Activation
Focal AT caused by abnormal automaticity is an arrhythmia arising from the distinctive site of earliest activation and propagating centrifugally to the rest of the atrium. The two major mechanisms of AT with abnormal automaticity are triggered activity and enhanced automaticity. Both these mechanisms of AT can be induced by catecholamines, but AT with triggered activity can be induced or terminated through programmed stimulation and is easily terminated by adenosine injection.40 Conversely, AT with enhanced automaticity can only be transiently suppressed through an adenosine injection and cannot be induced by programmed pacing.15 Focal AT has been found to account for 22.2% of ATs after PVI,41 and, in that study, all focal ATs were terminated by RF ablation at the site with earliest atrial activation, which were related to the conduction gaps around the PV ostium.41
Macroreentrant Atrial Tachycardia
Macroreentrant AT is the most common form of AT after AF ablation. Rostock et al. stated that 72% of ATs after ablation in persistent AF were macroreentrant.42 Moreover, Pascale et al. reported that more than half of recurrent arrhythmias were macroreentrant.43 The incidence of AT after AF ablation varies, mainly depending on the ablation strategy and lesions in the index procedure. These ATs may be easily induced but are difficult to terminate because of atrial anisotropy, scarring or gaps in previous ablation lesions, and multiloop reentrant circuits during AT. Atrial structural or electrical remodelling, both AF and ablation related, can lead to small amplitude EGMs, an obscure isoelectric line and undulating P-wave morphology, which make differentiating the origin of macroreentrant ATs difficult. Unlike focal ATs, macroreentrant ATs are insensitive to adenosine infusion.44
Microreentrant Atrial Tachycardias After PVI
Microreentrant ATs, also referred to as ‘localised reentry’ ATs, were described initially by Jais et al. and have since been reported by other groups.45,46 This subtype of AT was later defined as a circuit with the entire AT cycle length (CL) within a single atrial segment smaller than 2–3 cm, spreading centrifugally from the area of activation.39 This arrhythmia is noted predominantly in regions previously ablated or in those that contain extremely slow conduction allowing an extremely small circuit. Unlike focal ATs caused by abnormal automaticity, microreentrant ATs are insensitive to adenosine.47
Pulmonary Vein-Gap Reentrant Atrial Tachycardias
Another remarkable form of AT is PV-gap reentrant AT (PV-gap RATs), which is observed after PVI and is difficult to identify using conventional mapping. The circuits of PV-gap RATs involve both macroreentrant and microreentrant ATs and are associated with the prior ablation strategy. A case series determined that local reentrant ATs at the PV ostium after PVI were observed mostly in the right PV.41 Chang et al. showed that 70% of gap-related ATs after PVI were reentry ATs, with most of them related to the left PV.48 A multicentre study found that PV-gap RATs constituted 7% of all ATs after AF ablation.49 Using ultra-high-density mapping, PV-gap RATs were divided into the following three groups in the study: two gaps in one PV, two gaps in the ipsilateral superior and inferior PVs, and two separate gaps in one PV and the contralateral PVs with a long circuit. The P-wave morphology of PV-gap RATs indicated a positive or RS pattern in the lead V1 and an isoelectric interval in all leads, due to approximately 50% of the tachycardia CL being within the PV slow conduction zones. Ablation towards either the exit or entrance gap effectively terminated the PV-gap RAT.
Mapping and Ablation
Atrial Tachycardias Caused by Focal Activation
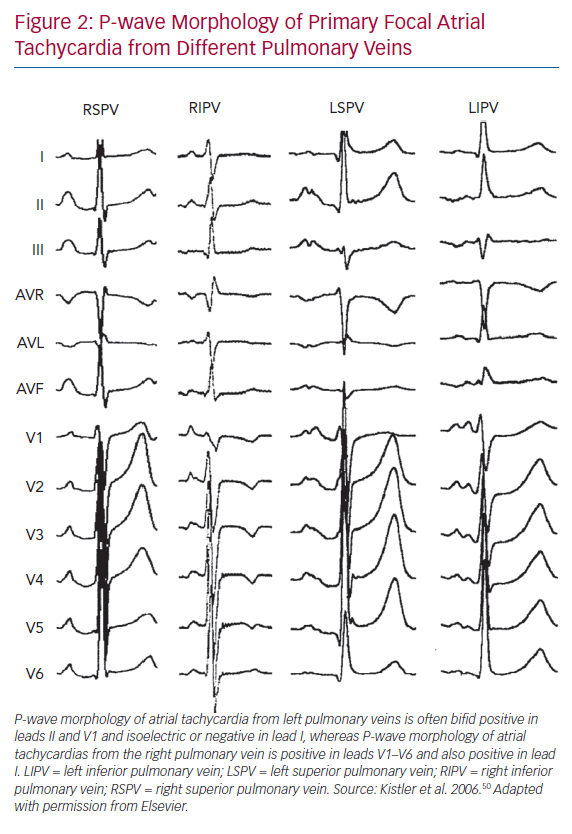
Kistler et al. determined in their large cohort study that more ATs due to abnormal automaticity arose from the RA than from the LA (73% versus 27%) in patients with primary focal ATs.50 The three most common locations of focal AT were found to be the crista terminalis (31%), tricuspid annulus (22%) and PV (19%).50 In that study, ATs arising from PVs were often present with a positive P wave throughout the precordial leads and inferiorly directed.50 Leads I, II and V1 are crucial for differentiating left and right PV origin. Amplitudes in leads II, III and aVF help distinguish between superior and inferior PVs. ATs from left PVs show a bifid-shape P wave in V1 (M shape) and negative P wave in lead aVL, whereas those from right PVs have a late-peaking positive P wave in lead V1 and flat or biphasic P waves in lead aVL (Figure 2). Conversely, Gerstenfeld et al. reported that the most common site of focal ATs after AF ablation was the previously isolated PV.17 Their findings were concordant with those of Ouyang et al., who found that PV tachycardia activated the atrium after continuous circular lesions.22 Generally, multipolar diagnostic catheters, such as decapolar or spiral catheters, can be used to determine the earliest activation site, with the earliest activation often preceding the onset of the P wave by 30 ms or more and with the unipolar EGMs indicated a QS pattern. Mohamed et al. reported the role of atrial overdrive pacing (AOP) in localising focal atrial tachycardia.51 In that small but elegant study, they found that the difference between post-pacing interval (PPI) and tachycardia CL (TCL; PPI-TCL) of focal ATs has a direct relationship to proximity of the pacing site to the tachycardia focus.51 Given that there was little perifocal tissue of focal ATs as compared with the sinus node, the PPI-TCL at the AT focus was usually less than 20 ms while the difference between PPI and sinus CL was above 80 ms. This manoeuvre is very useful in distinguishing AT close to the sinus node, such as ATs from the superior crista terminalis or lower superior vena cava, from sinus tachycardia.51 Electroanatomical mapping is also a useful tool for identifying the origin of focal ATs, which demonstrate centrifugal activation from a discrete point source. Additionally, it provides the information of substrate remodelling after previous ablations and helps electrophysiologists recognise gaps along the previous ablation line.
Catheter ablation can be performed at the earliest activation site using a non-irrigated or irrigated radiofrequency catheter. Acceleration of tachycardia during ablation suggests that the site is an excellent target. Regarding focal ATs from PVs conducting through gaps along the isolation line, which were typical origins after PVI, reisolation of a PV antrum with a bidirectional block has been suggested. Although focal ATs, which have the earliest site near the ostium, can be directly ablated, this procedure might increase the risk of PV stenosis. In regions close to the atrioventricular (AV) node, cryoablation is an alternative method to preventing AV block.
Macroreentrant Atrial Tachycardias
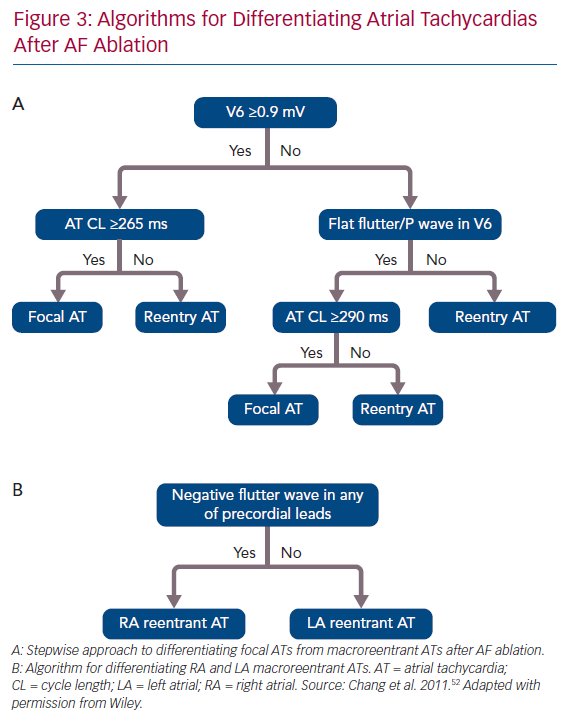
ECG features of a flutter wave remain a useful tool helping clinical electrophysiologists evaluate the possible mechanisms of and plan an ablation strategy for macroreentrant ATs. Chang et al. reported a stepwise algorithm for differentiating between focal and macroreentrant ATs after AF ablation (Figure 3).52 In that study, focal ATs had higher positive amplitudes of P or flutter waves in V6 and longer tachycardia CLs than macroreentrant ATs. Negative P or flutter waves that appeared in at least one precordial lead were more commonly seen in the RA than in the LA.52 Pascale et al. presented some ECG characteristics to help recognition of macroreentrant ATs.53 A negative P wave in the lead V1 suggested peritricuspid ATs. The precordial transition from upright to negative flutter waveforms identified anticlockwise peritricuspid ATs. A negative or negative–positive P wave in any of the leads V2–V6 in the absence of a precordial transition suggested perimitral ATs.53 Regarding the analysis of intracardiac EGMs during AT, the coronary sinus (CS) activation pattern is always the first step because it provides pertinent information on macroreentrant circuits. Simultaneous CS activation is a typical characteristic of roof-dependent ATs.43 Kim et al. reported that a CS activation time <45 ms combined with entrainment pacing from the roof and posterior wall both help in the differentiation between roof-dependent ATs and perimitral and CTI-dependent ATs.54 Casado Arroyo et al. stated that a CS activation time ≤39 ms can assist in the diagnosis of roof-dependent ATs with a sensitivity of 100% and specificity of 97%.55
Entrainment is a classical manoeuvre that can clarify whether sites participate in the circuits of ATs. Entrainment pacing from two or more different atrial segments, such as the CTI and proximal and distal CS, should be conducted initially to confirm whether the RA is involved in the circuit. If LA macroreentrant ATs are suspected, entrainment pacing should be performed from two opposite segments, namely the septal and mitral isthmus for perimitral ATs and the anterior and posterior walls for roof-dependent ATs. A PPI-TCL within 30 ms confirms that the site is in the circuit. Downstream overdrive pacing (DOP) and the identification of intracardiac concealed fusion (ICF), as reported by Barbhaiya et al., can help electrophysiologists locate the suspected reentrant circuits rapidly and facilitate catheter ablation.56 Initial DOP from the CS catheter was performed to confirm whether arrhythmias were perimitral ATs. If ICF was not identified during the DOP from the CS catheter, further DOP from the LA roof was achieved to clarify whether the circuit was roof dependent. Additional DOP from the posterior LA wall, anterior LA wall, LA appendage, LA septum and LA floor supported the further diagnosis of locally reentrant ATs and focal ATs. Approximately 80% of ATs could be accurately diagnosed after seven DOP attempts.
Although entrainment and overdrive pacing can help clinicians identify the circuit and critical isthmus of macroreentrant ATs, several limitations restrict their application. Sometimes, the entrainment pacing easily interrupts the ATs or transforms them into other tachycardias. Moreover, rapid burst pacing in the diseased myocardium can cause rate-dependent conduction delay, thereby misleading the clinician through a lengthy PPI.57 Detailed mapping of tachycardia circuits with the entire CL using electroanatomic systems can assist in verifying the mechanism of macroreentrant ATs. Missing segments of the entire tachycardia CL imply that areas of slow conduction with extremely low amplitude or fractionated EGMs are not annotated, or that part of the circuit is involved in another atrial segment or epicardial conduction through the CS.58–60 More recently, the novel ultra-high-density mapping technique has helped in identification of the mechanisms underlying complex arrhythmias. This technique allows a lower scar threshold setting and identifies critical isthmuses with less noise on the bipolar EGMs. Frontera et al. presented an excellent work regarding the EGM characteristics related to different electrophysiological mechanisms.59 In their study, EGMs at low conduction were of low amplitude, of long duration and fractionated, whereas those at wavefront collision had high amplitude, short duration and double or triple deflections with less fractionation. Gaps along the ablation line had narrowly spaced potentials with fractionation in between. The pivot sites consisted of pivot points with high-amplitude, short-duration and multiple-deflection EGMs, and friction sites presented with double potentials with low-amplitude and fractionated EGMs. Furthermore, Takigawa et al. delicately delineated the circuits of macroreentrant ATs after AF ablation using ultra-high-density mapping.61 They determined that the three conventional subtypes of macroreentrant ATs (i.e. peritricuspid, perimitral and roof-dependent ATs) could be further divided into different subgroups according to their propagation circuits. For example, perimitral ATs could be classified as the following three subgroups: type A, the circuit is equal to the entire mitral annulus (MA); type B, the circuit is larger than the entire MA with all MA included; and type C, the circuit is larger than the entire MA without the entire MA included. More than 90% of the practical isthmuses in type A (typical type) were on mitral isthmuses. However, only 50% of the practical isthmuses in types B and C were on mitral isthmuses. These critical regions included the septum, anterior wall, posterior wall, CS, ridge between the left PV and LA appendage, and CTI. Anatomic isthmuses used as ablation targets were significantly longer than the true, practical isthmuses exposed using high-density mapping.
Linear ablations between anatomical or electrical barriers, which interrupt the circuit, remain the essential tool in the management of macroreentrant AT ablation. Therefore, the atrial geometry, tachycardia reentrant circuits and gaps from the previous ablation should be clearly identified.13 Perimitral ATs are the most common reentrant ATs after AF ablation. They may be clockwise or anticlockwise along the MA, which is the anatomical obstacle for the reentrant circuit. The most common ablation line for the mitral isthmus is from the lateral MA to the left inferior PV. Because of the unstable catheter contact during mitral isthmus ablation, a deflectable long sheath is often used during the procedure. Some cases require epicardial ablation in the CS opposite the endocardial line to achieve a complete mitral isthmus block. Roof-dependent ATs are the second most common LA macroreentrant AT after AF ablation. The roof line can be achieved by ablating between the left and right superior PVs. In selected cases presenting with a figure-of-eight reentrant circuit around the mitral isthmus and through the roof, both mitral and roof lines should be performed. Another linear ablation strategy is LA anterior line, which connects the anterior mitral annulus to roof line or right superior PV, and it might be considered in cases of extensively diseased anterior LA with low amplitudes and conduction that could prompt small anterior re-entrant circuits.62 Because incomplete ablation lesions can lead to ATs in the future and should be avoided, a bidirectional conduction block must be confirmed after every linear ablation. Differential pacing, double potentials along the ablation line, or reobtaining the activation map after ablation can help confirm the existence of the bidirectional conduction block.62,63
Microreentrant Atrial Tachycardias After PVI
Conventional linear multielectrode catheters and 3D mapping systems provide limited information regarding microreentrant ATs because of their low resolution. Using ultra-high-density mapping, Frontera et al. identified localised atrial reentrant circuits with multiple slow conduction isthmuses in low-voltage areas. These circuits were around a fixed scar or line of conduction block.64 However, wavefront collision or artefacts can mimic microreentrant ATs, thereby leading to misinterpretation of the tachycardia circuit.65 Entrainment pacing from at least two separate points along the circuit should be performed to confirm whether these microreentrant ATs are active or passive in the atrial arrhythmias.
Successful ablation can be performed towards the long-duration and low-voltage fractionated EGMs in the circuit, which might be the sites of slow conduction isthmuses.59 However, not every slow conduction site lies in the critical isthmus. For example, EGMs of friction areas near the pivot point or wavefront collision also present a fractionated pattern and were found to be passively involved in the tachycardias.59 Therefore, careful interpretation of intracardiac EGMs and accurate delineation of tachycardia circuits are mandatory before ablation.
Procedure Outcome After Catheter Ablation
The mechanisms of AT, duration of AF and abnormal substrates, such as prior ablation lesions or existing atrial scars, affect the acute success and recurrence rates of catheter ablation of ATs. In focal ATs without previous AF ablation, the acute success rate has been found to be higher than 80% and the recurrence rate to be 4–14%;66,67 however, recurrent atrial arrhythmias after ablation of isolated LA ATs were not uncommon in one study and half of them were AF.68 Chae et al. reported that acute procedural success was achieved in 86% of ATs after circumferential PVI.28 Furthermore, catheter ablation was effective in terminating 96 of 116 macroreentrant ATs (83%), 18 of 18 focal ATs (100%) and 20 of 21 microreentrant ATs (95%). However, 27% of patients developed recurrent AT during follow up and approximately 40% had perimitral or roof-dependent ATs.28 In addition, similar results were reported in another study; despite using ultra-high-density mapping, a significant recurrence rate (26%) of macroreentrant ATs after AF ablation was observed and reconnection across the roof line and mitral isthmus was noted in most of the cases.61
Conclusion
Catheter ablation has become the most common non-pharmacological therapy of AF in the last decade and has increased the incidence of ATs after AF ablation, causing severe clinical problems because these ATs are typically symptomatic and drug refractory. Preventing unnecessary ablation lesions in the index procedure could prevent creation of a substrate for arrhythmogenesis. Because prior AF ablation, atrial remodelling and existing atrial myopathy alter the normal conduction of atria, limitations exist when using ECG features, intracardiac tracings or entrainment pacing to identify the circuit and accurately localise the critical isthmus. With the development of ultra-high-density mapping and novel annotation systems of electroanatomic mapping systems, clinical electrophysiologists can derive detailed information regarding the abnormal substrate and critical circuits of ATs and effectively perform ablations without causing iatrogenic arrhythmias.
Clinical Perspective
- There are three mechanisms of atrial tachycardia (AT) after AF ablation: AT caused by focal activation (triggered activity or enhanced automaticity), macroreentrant AT and microreentrant AT. These mechanisms are associated with previous catheter ablation strategy, surgical intervention and abnormal atrial substrates, such as scars.
- Macroreentrant ATs are the most common ATs after AF ablation. A complete bidirectional block across the ablation line at the conduction isthmus is mandatory to prevent iatrogenic tachycardias in the future.
- Microreentrant ATs are not uncommon after AF ablation and can sometimes be misinterpreted as focal ATs because of abnormal automaticity. Entrainment pacing can help confirm whether the circuit is active in the AT.
- Ultra-high-density mapping can help electrophysiologists recognise the tachycardia circuit and locate the critical isthmus accurately. Although long-duration and low-amplitude electrograms represent slow conduction in the circuit, not all of them are within the isthmus.
- Pulmonary vein-gap reentrant ATs are interesting iatrogenic tachycardias after pulmonary vein isolation. They can be cured using ablation targeting the entrance or exit gaps with the assistance of high-density mapping.