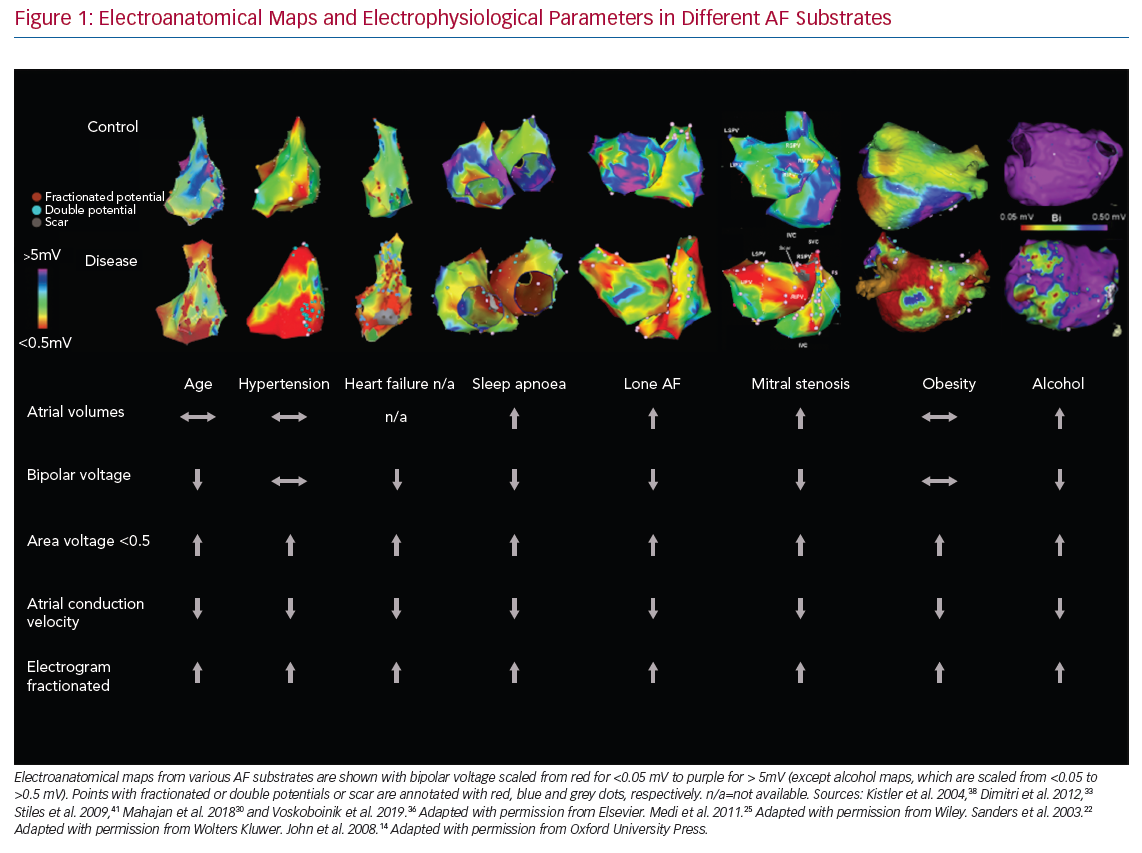
AF is the most common sustained cardiac rhythm disorder and is associated with increased morbidity and mortality. Since the first description of AF initiation by triggers from pulmonary veins sleeves, pulmonary vein isolation (PVI) has become the standard ablation strategy in patients with AF.1 However, freedom from the arrhythmia, particularly in non-paroxysmal AF, remains suboptimal, and it is now clear that, in these patients, AF is maintained by an atrial substrate beyond the pulmonary veins. Although electrical remodelling may be reversible with termination of the arrhythmia, the development of atrial substrate due to fibrosis contributes to the progression of the AF phenotype from paroxysmal to persistent AF, leading to an arrhythmia that is more refractory to intervention.2 It is clear from animal and human studies that prolonged AF can cause this structural change. Moreover, it is also apparent that a range of risk factors associated with AF, including age, obesity, heart failure (HF), valvular heart disease, hypertension (HT), sleep apnoea and alcohol intake, may also progress atrial remodelling. The rise in the prevalence of cardiovascular risk factors (particularly driven by ageing populations and the obesity epidemic) has been associated with an increase in the prevalence of AF and AF-related hospitalisations.3 In this review, we focus on insights from electrophysiological mapping studies in cohorts with AF risk factors. We discuss substrate mapping and its implications for AF management and outcomes, and also focus on potential pitfalls.
The Second Factor: Structural Remodelling is Required for AF Maintenance
Early studies of animal models have demonstrated that AF promotes acute electrical remodelling, which in turn leads to further AF, thereby introducing the seminal concept that ‘AF begets AF’.4–6 In response to either induced AF or rapid atrial pacing, a reduction in the atrial effective refractory period (ERP) occurs with an increase in the spatial heterogeneity of ERP and loss of normal ERP rate adaptation, all resulting in progressively longer durations of AF. In this model, termination of the arrhythmia results in remodelling reversal, suggesting that sinus rhythm may beget sinus rhythm. However, human studies of early intervention to re-establish sinus rhythm do not fully support this concept; the re-establishment of sinus rhythm has not been found to prevent the progression of AF in the majority of patients.7,8 Ongoing work has indicated that, beyond acute electrical remodelling, structural remodelling also occurs and is not necessarily fully reversible. This so-called second factor has been shown to occur as a result of longer durations of AF. However, the multiple conditions associated with AF also appear to promote significant structural remodelling.
Abnormal Atrial Substrates and Structural Remodelling in Conditions Predisposing to AF
It is well known that certain cardiac conditions and risk factors (i.e. age, obesity, HT, HF, structural heart disease, sleep apnoea and alcohol intake) are associated with AF, likely through both different and interacting mechanisms. In the next section, we review the evidence describing the nature of atrial structural remodelling in these conditions, even prior to the development of AF (Figure 1).
The Role of Atrial Stretch
The impact of acute atrial stretch on electrical remodelling has been studied in animal models and in humans. Despite variability in the reported effect on atrial ERP, evidence from these studies consistently demonstrates conduction slowing, conduction block and increased frequency of AF.9–11 In studies of atrial stretch related to loss of atrioventricular (AV) synchrony, Sparks et al. demonstrated evidence of both electrical and mechanical remodelling.12,13 Although refractoriness showed a variable increase, there was conduction slowing, sinus node impairment and a decrease in parameters of atrial contractile function. These changes developed over 3 months and were fully reversible with the return of AV synchrony.
Valvular and Congenital Heart Disease
The nature of atrial remodelling due to pressure and volume overload associated with either valvular heart disease or congenital heart defects has been studied for a number of different pathologies. Common to these is the development of significant atrial dilatation, creating one of the critical determinants for the maintenance of AF and structural remodelling.
In mitral stenosis, John et al. demonstrated the presence of abnormal atrial structural and electrical substrate in patients with symptomatic mitral stenosis referred for balloon valvuloplasty when compared to a control cohort.14 Biatrial mapping demonstrated significantly reduced biatrial voltage, reduced conduction velocity and prolonged ERPs. As expected, patients with mitral stenosis were more susceptible to AF induction with programmed extra stimuli. Such remodelling was found to be more profound in the left atrium (LA) than the right atrium (RA). Balloon valvuloplasty resulted in significant improvements in conduction and in bipolar voltage, either acutely or by 3 months of follow-up, indicating that even chronic remodelling may be, in part, reversible.15
Roberts-Thompson et al. studied patients with symptomatic mitral regurgitation referred for valve repair using epicardial plaque mapping in the operating room.16 Their high-density mapping study demonstrated the presence of conduction slowing, conduction heterogeneity with regions of conduction slowing and lines of block particularly in the posterior left atrium. Patients with mitral regurgitation had more advanced remodelling than a comparison group with normal mitral valves undergoing coronary bypass surgery.
Morton et al. performed right atrial mapping in patients with atrial septal defect (ASD) before and late after surgical closure.17 Electroanatomic maps of the RA demonstrated the presence of atrial conduction abnormalities, both generally and at the crista terminalis, sinus node dysfunction and atrial dilation, when compared to controls. In their study, closure of the ASD, while associated with significant reduction in atrial size, did not lead to recovery of conduction abnormalities, indicating partial reverse remodelling in this population. In a subsequent study, Roberts-Thomson et al. demonstrated similar atrial remodelling in the LA of ASD patients, indicating that the remodelling process is not confined to the RA in an ASD population.18
Congestive Heart Failure
The interaction between HF and AF has been studied in both animals and humans. Li et al. described remodelling ‘of a different sort’ in a canine model of ventricular tachypacing.19 This was characterised by an increase in conduction heterogeneity associated with interstitial fibrosis that resulted in an increase in AF inducibility. Other studies have demonstrated similar findings.20 Five weeks after HF reversal, neither fibrosis nor AF inducibility were found to demonstrate significant resolution.21
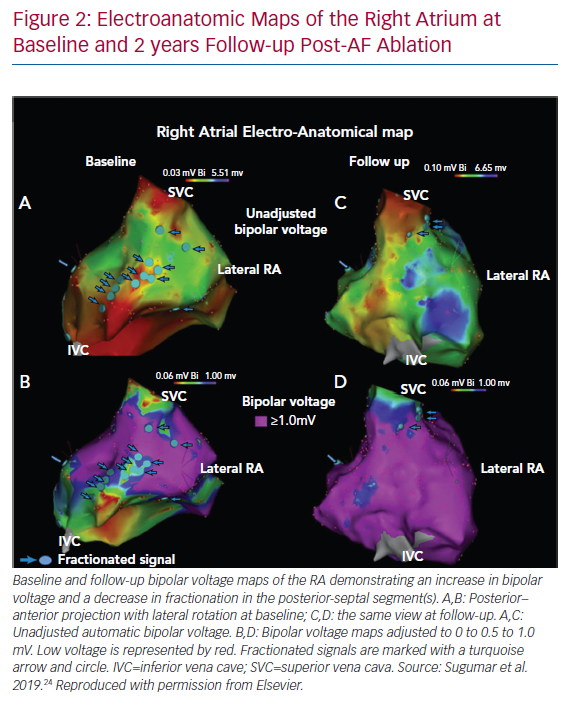
Sanders et al. demonstrated slowing of atrial conduction, low-voltage areas (LVAs), a greater number of fractionated electrograms and abnormal sinus node function in patients with congestive HF (CHF) when compared to a control population.22 Patients with CHF demonstrated increased AF inducibility. More recently, Prahbu et al. performed high-density electroanatomic mapping of both atria in two cohorts of patients: persistent AF with normal left ventricular systolic function (left ventricular ejection fraction [LVEF] >55%) and persistent AF with idiopathic cardiomyopathy (LVEF <45%).23 HF was associated with significantly reduced biatrial bipolar tissue voltages, greater voltage heterogeneity and significantly more biatrial electrogram fractionation compared to no HF, suggesting the impact of HF on structural remodelling above and beyond the effect of AF itself. When patients were restudied 2 years after catheter ablation with maintenance of sinus rhythm and significant improvement or normalisation of LV function, remodelling reversal was found to be incomplete.24 There was a reduction in complex signals and patchy regional increases in voltage, but no improvement in atrial conduction, again indicating that advanced remodelling is unlikely to reverse (Figure 2).
Systemic Hypertension
Medi et al. performed RA electroanatomic mapping in patients with HT (but no AF) and in controls.25 HT was associated with extensive conduction abnormalities, particularly in the posterior RA at the crista terminalis. In addition, an increased number of LVAs and AF inducibility were noted, despite prolonged ERPs compared to controls. To the best of our knowledge, no studies have evaluated the impact of HT on LA remodelling; however, studies in patients with HF and preserved ejection fraction are ongoing.
Obesity
Emerging data have indicated that obesity and increased pericardial fat are associated with a more advanced atrial substrate. A number of animal studies have demonstrated the impact of progressive weight gain on the atrial substrate and inducibility of AF.26,27 Abed et al. demonstrated the progressive change in electrical and structural remodelling in a group of 30 sheep fed a high-calorie diet over an 8-month period.26 Increasing weight was associated with increasing LA volume, fibrosis, upregulation of inflammatory markers, decreased conduction velocity and an increase in conduction heterogeneity. Such changes were found to be associated with an increase in both inducible and spontaneous AF. In a subsequent study, the same group demonstrated that these changes were most marked in the posterior left atrium and were associated with fat infiltration and fibrosis. Other animal studies noted that a high-fat diet could increase AF duration due to slow atrial conduction and reduced pulmonary vein refractoriness without necessarily accompanying obesity.28,29 Mahajan et al. compared atrial electroanatomic maps and epicardial adipose tissue in obese patients with the same data from a non-obese cohort.30 Obesity was found to be associated with an increase in all measures of epicardial adipose tissue (EAT), with a predominant distribution adjacent to the posterior left atrium and the atrioventricular groove. Obese patients had reduced global conduction velocity, increased fractionation and increased LVAs. LVAs were predominantly seen in the posterior and/or inferior LA, matching the location of EAT on cardiac magnetic resonance (CMR) imaging. Another study of the impact of obesity on atrial remodelling also found significant conduction slowing at the pulmonary vein-to-LA junction.31
Obstructive Sleep Apnoea
Obstructive sleep apnoea (OSA) is known to be associated with AF.32 Dimitri et al. characterised the atrial substrate among a cohort of patients undergoing AF ablation who either had OSA (apnoea hypopnea index >15) or no OSA.33 Patients with OSA had lower atrial voltage, prolonged conduction times and greater percentage of complex fractionated electrograms, but there was no difference in atrial ERP. Similar findings were recently described by Anter et al. in their cohort of 86 patients (n=43 with OSA and n=43 without OSA) undergoing PVI for paroxysmal AF.34 However, they also reported a higher prevalence of non-pulmonary vein triggers in OSA compared to controls, indicating that the development of AF in this population may be due to autonomically mediated triggers interacting with chronic substrate.
Alcohol
Alcohol has recently emerged as an important modifiable risk factor in AF, and both binge and habitual drinking seem to increase the vulnerability to AF through its impact on atrial remodelling. Qiao et al. performed voltage mapping in 122 patients undergoing PVI for paroxysmal AF, and classified them according to their daily alcohol consumption history.35 Heavy drinkers had more LVAs and more AF recurrences compared to moderate drinkers and alcohol abstainers. Importantly, both heavy alcohol consumption and LVAs were independent predictors of AF recurrence. Similar findings were recently found in Voskoboinik et al.’s study of the atrial substrate among alcohol consumers.36 The authors performed high-density electroanatomic mapping of the LA in 75 patients undergoing PVI for AF. Patients were classified as lifelong non-drinkers, mild drinkers (2–7 drinks/week) and moderate drinkers (8–21 drinks/week). When compared to alcohol abstinence or mild alcohol consumption, moderate alcohol consumption was associated with significantly lower global atrial voltage, slower conduction velocities and an increased proportion of both complex atrial potentials and LVAs. Moderate drinking, together with age and female sex, were found to be independent predictors for low voltage. Ongoing studies are addressing the question of whether atrial remodelling may reverse with abstinence, and a recent randomised study indicated that abstinence reduces AF recurrence.37
Age
It is well known that the prevalence of AF increases with age. A number of animal and human studies have demonstrated the presence of an abnormal electrical substrate in older cohorts, independent of changes in the atrial ERP.38–40 Kistler et al. performed high-density electroanatomic mapping of the RA in 41 patients with no history of AF.38 Patients were stratified into three groups according to age: <30 years, 30–60 years and >60 years. Ageing was associated with regional conduction slowing, anatomically determined conduction delay at the crista terminalis, areas of low voltage, impaired sinus node function, and an increase in atrial ERP.
Lone AF and Fibrotic Cardiomyopathy
The term ‘lone AF’ has been variably defined over decades of use, but broadly, can be taken to imply AF in the absence of structural heart disease or HT. In a detailed mapping study, Stiles et al. demonstrated the presence of abnormal atrial substrate in patients with paroxysmal lone AF compared to a control group, even when studied distant to an AF episode.41 Paroxysmal AF patients were found to have atrial dilatation, lower mean voltage, prolonged atrial conduction times, impaired sinus node function and increased atrial ERP compared to the control group. However, in their study, more contemporary causes of atrial remodelling, such as obesity, sleep apnoea and alcohol intake, were not clearly excluded, and it is possible that the observed remodelling reflected the high prevalence of these conditions in an apparent lone AF population. An alternate hypothesis is that AF is secondary to a primary underlying fibrotic cardiomyopathy, as proposed by Kottkamp et al.42 Human histological and imaging studies evaluating fibrosis in lone and non-lone AF populations have found comparable fibrosis distribution between the two groups,43,44 supporting the idea of a primary fibrotic cardiomyopathy. However, a detailed exclusion of the above causes of remodelling was not performed.
How Best to Define Abnormal Atrial Substrate: Pitfalls
On electroanatomic mapping, the assumption inherent in the findings of low voltage, slowed conduction and complex signals is that structural change is present, the hallmark of which is fibrosis.45 Human histological studies have confirmed the presence of atrial fibrosis in patients with AF, and the fibrosis extent correlated with AF duration.43,46,47
However, to date, there is no direct correlation confirming the relationship between atrial substrate on mapping and histological fibrosis. The widely applied definition of abnormal atrial bipolar voltage (<0.5 mV representing LVAs and <0.05 mV representing scar) derives from mapping with an ablation catheter bipole and has never been fully validated in humans.22 The indices have been used to successfully predict outcomes when used to define LVA in patients with paroxysmal or persistent AF.48–50 Furthermore, complex fractionated electrograms and abnormal conduction during sinus rhythm tend to correspond to LVAs, suggesting that they may represent areas of histological fibrosis.51,52 However, multiple potential pitfalls exist when simply using voltage as a marker of atrial remodelling and fibrosis. For example, atrial wall thickness varies markedly between different atrial regions (e.g. trabeculated compared with smooth-walled atrium), and also from patient to patient. It is highly probable that normal voltage also varies considerably and may defy a simple cut-point definition. Moreover, these values were described when using an ablation bipole for mapping. The start of the century has witnessed the introduction of multielectrode mapping catheters for electroanatomic mapping.53 Compared with linear, single-point conventional mapping catheters, multielectrode mapping catheters have the combination of smaller electrode size, smaller interelectrode distance and multiple splines. This allows for recording electrograms from a significantly smaller underlying tissue diameter with multiple orientations. This translates to higher mapping resolution that can identify heterogeneity within the area of low voltage, localising channels of surviving bundles. Moreover, the smaller electrode and closer interelectrode spacing means less signal averages and cancellation effects, which may translate to higher recorded bipolar voltage amplitude with shorter electrogram duration.53,54 However, the criteria for bipolar low voltage using multielectrode mapping catheters has not been systematically revisited.
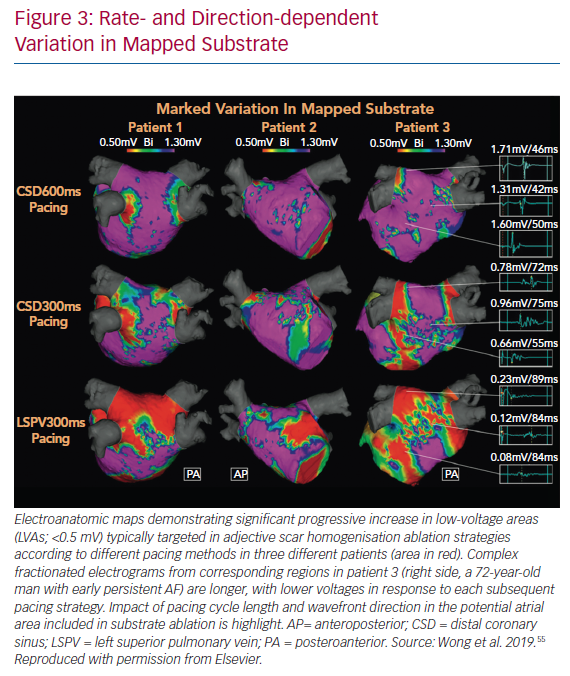
It is also important to note that both the distribution and extent of LVAs on electroanatomic mapping is critically dependent on the directionally and rate of wavefront prorogation. Wong et al. demonstrated that a change in pacing site or cycle length could change the region defined as low voltage by up to 30% (Figure 3).55
The nature of the rhythm is also of critical importance. Several early studies demonstrated that regions of low bipolar voltage and complex fractionated electrograms recorded during AF frequently correspond with areas of normal atrial bipolar voltages in sinus rhythm.52,56 However, recent studies using omnipolar mapping, indicating that electrode orientation is a key determinant of recorded bipolar voltage, have raised the possibility that mapping during AF may provide an improved evaluation of underlying substrate. In an elegant study using omnipolar mapping with a grid catheter, Haldar et al. showed that bipole orientation has a significant impact on bipolar electrogram (EGM) voltages obtained during sinus rhythm (SR) and AF.57 In that study, omnipolar EGMs were able to extract maximal voltages from AF signals not influenced by directional factors, wavefront collision or fractionation. Other techniques to identify substrate have focused on signal characteristics. Approaches beyond the traditional identification of complex fractionated electrograms have included targeting spatio-temporal dispersion of electrograms; targeting regions of prolonged and continuous fractionation; various approaches to determine the site of highest activation frequency, such as dominant frequency; or using activation gradients, such as in the stochastic trajectory analysis of ranked signals mapping approach.58–61
In addition to mapping techniques, multimodality imaging can provide a non-invasive assessment of the abnormal atrial substrate in patients with AF.62 In addition to atrial size and morphology, mechanical and structural remodelling parameters can be obtained via strain imaging and late gadolinium enhancement CMR imaging (LGE-MRI). LGE-MRI has been proposed as a more effective way in which to identify regions of atrial fibrosis, and a considerable body of work indicates that this may be feasible. However, not many departments have been able to replicate these data, particularly for the reliable identification of more subtle interstitial fibrosis.62 A key problem is how to accurately define the number of standard deviations (SD) from the mean reference signal intensity, which most closely describes accurate scar volume. In an animal ablation study, the point at which CMR imaging and histological scar volume were equal was in the steepest portion of the graph, which meant any small change in SD (chosen by definition) would create a corresponding large difference between CMR imaging and histological measured volumes.63 Chen et al. studied the correlation between delayed enhancement on CMR imaging and LVAs on electroanatomic mapping in 16 patients with persistent AF.64 There was a mismatch between delayed enhancement areas and LVAs; delayed enhancement was present in 61% of LVAs, whereas low voltage was present in 28% of delayed enhancement areas. In another recent multimodal examination of AF substrate, Zghaib et al. demonstrated that LGE-MRI, high-density mapping and point-by-point mapping with the ablation catheter demonstrated good correlation in delineating electroanatomical AF substrate, providing some enthusiasm for the routine use of CMR imaging.65 Given the current challenges in technique and reproducibility, and the lack of prospective studies, the current role for LGE-MRI in the management of patients with non-paroxysmal AF remains limited to a relatively small number of centres with extensive experience in the technique. Data from the prospective Delayed-Enhancement MRI Determinant of Successful Radiofrequency Catheter Ablation of Atrial Fibrillation-II (DECAAF-II) study are eagerly awaited.62
Implications of Accurately Identifying the Atrial Substrate
Numerous studies have indicated that advanced atrial substrate is a risk factor for recurrence following AF ablation.48,49,66,67 In addition, preliminary and observational studies suggest that isolation or homogenisation of these abnormal regions significantly improves post-ablation arrhythmia-free survival.59,69,70 Schreiber et al. implemented the concept box isolation of fibrotic areas and studied its impact when added to traditional PVI on AF-free survival among 92 patients with fibrotic atrial cardiomyopathy, as defined by voltage mapping.71 This approach was associated with a 69% arrhythmia-free survival at 16 ± 8 months. In a single-centre randomised study, Kircher et al. examined whether targeting LVAs in addition to PVI was more effective than PVI plus linear ablation in patients with paroxysmal and persistent AF.72 At 12 months, the LVA ablation group had better arrhythmia-free survival compared to the PVI plus linear ablation group (68% versus 42%, p=0.003). More recently, Yang et al. randomised 229 patients with non-paroxysmal AF to either low-voltage, zone-guided ablation or standard stepwise ablation (including linear ablation), but did not show a significant improvement in arrhythmia-free survival at 18 months (74% versus 71%, p=0.32).73 However, procedure times, total ablation time and fluoroscopy times were significant shorter using the LVA-guided approach. Further multicentre studies are needed to better define the role of LVA-guided substrate ablation in the management of patients with persistent AF. A prospective multicentre randomised study is currently ongoing to examine the efficacy of atrial fibrosis (based on MRI-LGE)-guided ablation intervention in the treatment of patients with persistent AF (DECAAF-II, NCT02529319).
Progression and Regression of the Atrial Substrate
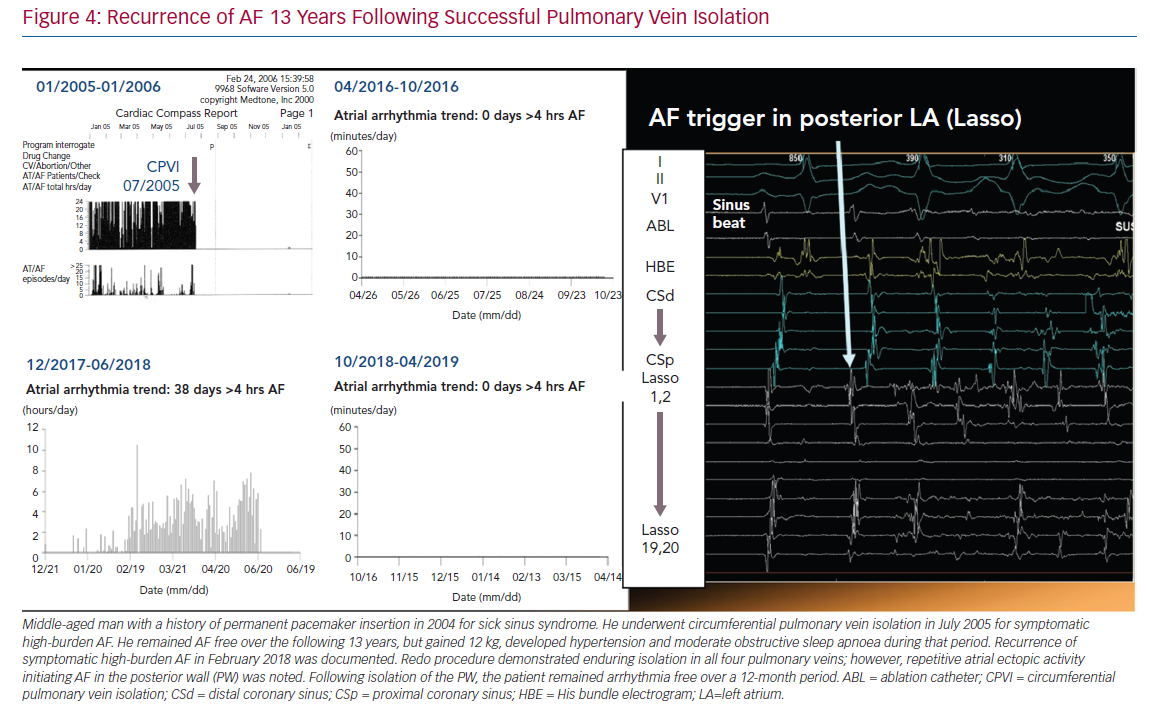
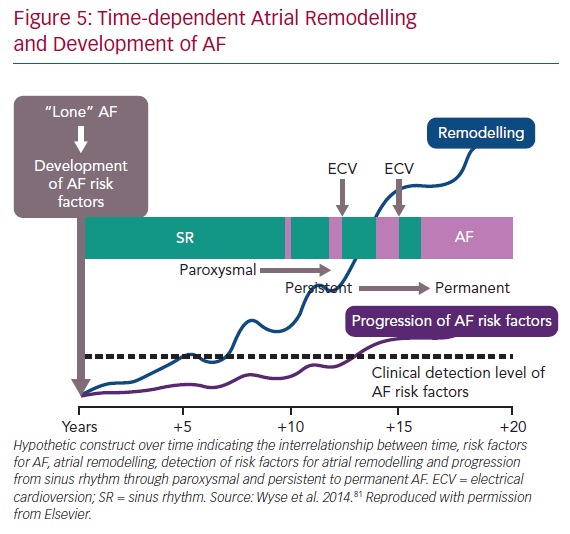
In many patients with AF, there is gradual progression from short-lasting paroxysmal AF to more frequent and persistent AF (Figure 4).74,75 This progression is, at least in part, driven by the evolution of the atrial substrate as a result of underlying risk factors and the arrhythmia itself. As such, the hypothesis emerged that both risk factor management and rhythm control might arrest the progression and perhaps reverse the remodelling of the atrial substrate in AF (Figure 5).76,77 The data are mixed. Animal studies of structural remodelling reversal have shown variable results, but established replacement fibrosis has not resolved.78 In humans, AF ablation did not result in reverse remodelling at 6 months, with some evidence of further progression.79 Studies of risk factor management have indicated a significant propensity for reverse remodelling in animal studies. In humans, risk factor management has resulted in fewer recurrences of AF after ablation, and reversal of AF progression.37,76,80 Patients with weight loss frequently regress from a persistent to paroxysmal phenotype and progress in the opposite direction much less frequently.82
Conclusion
The abnormal atrial substrate plays a key role in the perpetuation of AF. While developing an ablation strategy targeting the atrial substrate seems logical, the current mixed results may reflect uncertainties as to how best to identify the critical arrhythmogenic substrate. Future improvements in mapping and imaging technology will certainly improve our understanding of the atrial substrate, and potentially pave the way for the development of tailored ablation therapies to improve arrhythmia outcomes.
Clinical Perspective
- Structural remodelling plays an important role in the development and clinical progression of AF.
- Beyond the impact of AF itself on structural remodelling, multiple associated conditions contribute to the development of abnormal atrial substrate.
- Further improvements in current atrial substrate imaging and mapping modalities are required to improve our understanding of structural remodelling to better guide substrate-based ablation strategies.
- Promising emerging work suggests an important role for risk factor management in arresting or reversing atrial remodelling and in improving AF outcomes. Further work is required to define the broader efficacy of this approach.